Abstract
Background
Convalescent plasma has been one of the most common treatments for COVID-19, but most clinical trial data to date have not supported its efficacy.
Research Question
Is rigorously selected COVID-19 convalescent plasma with neutralizing anti-SARS-CoV-2 antibodies an efficacious treatment for adults hospitalized with COVID-19?
Study Design and Methods
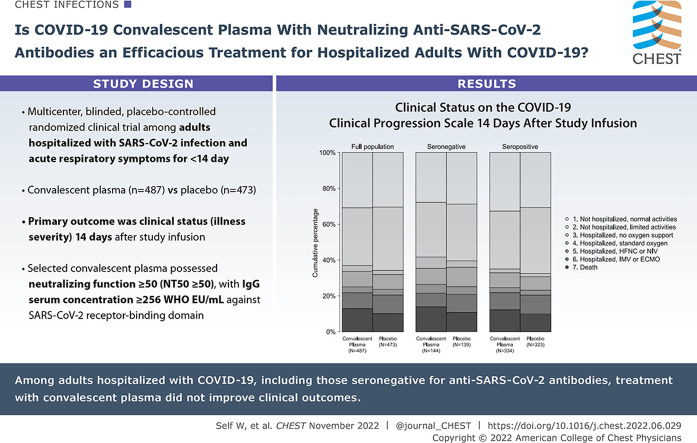
This was a multicenter, blinded, placebo-controlled randomized clinical trial among adults hospitalized with SARS-CoV-2 infection and acute respiratory symptoms for < 14 days. Enrolled patients were randomly assigned to receive one unit of COVID-19 convalescent plasma (n = 487) or placebo (n = 473). The primary outcome was clinical status (disease severity) 14 days following study infusion measured with a seven-category ordinal scale ranging from discharged from the hospital with resumption of normal activities (lowest score) to death (highest score). The primary outcome was analyzed with a multivariable ordinal regression model, with an adjusted odds ratio (aOR) < 1.0 indicating more favorable outcomes with convalescent plasma than with placebo. In secondary analyses, trial participants were stratified according to the presence of endogenous anti-SARS-CoV-2 antibodies (“serostatus”) at randomization. The trial included 13 secondary efficacy outcomes, including 28-day mortality.
Results
Among 974 randomized patients, 960 were included in the primary analysis. Clinical status on the ordinal outcome scale at 14 days did not differ between the convalescent plasma and placebo groups in the overall population (aOR, 1.04; one-seventh support interval [1/7 SI], 0.82-1.33), in patients without endogenous antibodies (aOR, 1.15; 1/7 SI, 0.74-1.80), or in patients with endogenous antibodies (aOR, 0.96; 1/7 SI, 0.72-1.30). None of the 13 secondary efficacy outcomes were different between groups. At 28 days, 89 of 482 (18.5%) patients in the convalescent plasma group and 80 of 465 (17.2%) patients in the placebo group had died (aOR, 1.04; 1/7 SI, 0.69-1.58).
Interpretation
Among adults hospitalized with COVID-19, including those seronegative for anti-SARS-CoV-2 antibodies, treatment with convalescent plasma did not improve clinical outcomes.
Clinical Trial Registration
ClinicalTrials.gov; No.: NCT04362176; URL: www.clinicaltrials.gov
Key Words: convalescent plasma, COVID-19, passive immunity, SARS-CoV-2
Graphical Abstract
Take-home Points.
Study Questions: Is COVID-19 convalescent plasma an efficacious therapy for improving clinical outcomes among adults hospitalized with respiratory symptoms from COVID-19 when administered within 14 days of symptom onset?
Results: In this multicenter, blinded, placebo (lactated Ringer’s)-controlled randomized trial among 960 adults hospitalized with COVID-19, patients treated with one unit of convalescent plasma shortly after hospital admission had nearly identical clinical outcomes compared with those treated with placebo, including illness severity on the World Health Organization COVID-19 Clinical Progression Scale 14 days after treatment (aOR, 1.04; 1/7 SI, 0.82-1.33) and 28-day mortality (aOR, 1.04; 1/7 SI, 0.69-1.58).
Interpretation: Among adults hospitalized with COVID-19, treatment with neutralizing COVID-19 convalescent plasma did not improve clinical outcomes.
SARS-CoV-2 caused approximately 450 million cases of COVID-19 and 6 million deaths worldwide during the first 2 years of the COVID-19 pandemic.1 , 2 Since the beginning of the pandemic, passive immunity, including the use of convalescent plasma, has been advanced as a potentially promising approach for treating COVID-19.3, 4, 5, 6
The rationale for using COVID-19 convalescent plasma relies on the concept of transferring anti-SARS-CoV-2 antibodies from a person who recently recovered from COVID-19 to another person who is in the early stages of infection and has not fully developed his or her own immune response.7 Based on strong biological rationale, COVID-19 convalescent plasma has been widely used during the pandemic.6 However, most published clinical trial data suggest that COVID-19 convalescent plasma is not efficacious for the most severely ill, hospitalized patients.8, 9, 10, 11, 12, 13, 14
The reasons why prior trials failed to show benefit for convalescent plasma among hospitalized patients with COVID-19 are not conclusively known. If COVID-19 convalescent plasma is beneficial for some patients hospitalized with COVID-19, two potential explanations that may have contributed to null findings in prior trials include: (1) wide variability in the quality (neutralizing activity) of the convalescent plasma used; and (2) inclusion of patients who had already established their own immune response to SARS-CoV-2. Only convalescent plasma with neutralizing anti-SARS-CoV-2 antibodies would be expected to potentially have efficacy. Most prior COVID-19 convalescent plasma trials used antibody-binding assays to select plasma with anti-SARS-CoV-2 antibodies.8, 9, 10 , 14 However the presence of antibodies does not guarantee neutralizing activity,15 and most trials did not confirm neutralizing activity of the transfused convalescent plasma.8, 9, 10 , 14 Furthermore, although passive immunity therapies are most likely to be efficacious among patients without an endogenous anti-SARS-CoV-2 immune response, many patients hospitalized with COVID-19 have an endogenous anti-SARS-CoV-2 response by the time of hospital admission,16 , 17 and many COVID-19 convalescent plasma trials did not evaluate for efficacy in a population restricted to those without endogenous antibodies.8 , 9 Therefore, this trial, Passive Immunity Trial for Our Nation (PassITON), was conducted to test the hypothesis that COVID-19 convalescent plasma with laboratory-confirmed anti-SARS-CoV-2 neutralizing activity improves clinical outcomes when administered to adults hospitalized with COVID-19 and to provide separate efficacy estimates for patients who did and did not have endogenous anti-SARS-CoV-2 antibodies prior to treatment.
Study Design and Methods
Trial Design and Oversight
The rationale and design of this trial have been previously published18 and are available in the protocol and statistical analysis plan included as Supplemental Data. We conducted a multicenter, blinded, placebo-controlled, randomized clinical trial to study the efficacy and safety of COVID-19 convalescent plasma as a treatment for adults hospitalized with COVID-19. Patients were enrolled at 25 US hospitals (e-Table 1) between April 28, 2020, and June 1, 2021. The trial started as a single center study at Vanderbilt University Medical Center with funding from the Dolly Parton COVID-19 Research Fund and then expanded to a multicenter study in September 2020 with funding from the National Center for Advancing Translational Sciences (NCATS). A central institutional review board at Vanderbilt University Medical Center approved the study. An independent Data and Safety Monitoring Board provided trial oversight. COVID-19 convalescent plasma was used in the trial under US Food and Drug Administration Investigational New Drug number 21080. Participants or legally authorized representatives provided written informed consent prior to trial participation.
Patient Population
We enrolled adults hospitalized with laboratory-confirmed SARS-CoV-2 infection and respiratory symptoms consistent with COVID-19 for < 14 days. These individuals included patients hospitalized on general medical floors and in ICUs, including those receiving no oxygen therapy, standard oxygen therapy, high-flow oxygen therapy, noninvasive ventilation, or invasive mechanical ventilation. Major exclusion criteria were prior COVID-19 vaccination, use of a COVID-19 passive immunity therapy in the prior 30 days, and contraindication to blood product transfusion. Full eligibility criteria are listed in e-Methods Section A.
Randomization and Blinding
Using a centralized, web-based Research Electronic Data Capture platform,19 enrolled patients were randomized in a 1:1 ratio to receive COVID-19 convalescent plasma or placebo stratified according to site, sex, and age. The placebo solution was lactated Ringer’s with multivitamin additives, which matched the color of plasma, and the study product was covered with masking bags during infusion. To safely administer blood products and maintain blinding of study group assignment, the trial used both blinded and unblinded study personnel at each site. Patients, investigators, outcome assessors, and treating providers remained blinded. Unblinded personnel included the study team member who randomized the patient and personnel in the blood bank and investigational pharmacy (e-Methods Section B).
Trial Intervention
Most of the convalescent plasma used in this trial was collected by trial-specific plasma donation drives at Vanderbilt University Medical Center in collaboration with Blood Assurance between April 22, 2020, and April 3, 2021. During the single-center component of the trial (prior to October 1, 2020), convalescent plasma units were selected based on serum concentration of IgG against the SARS-CoV-2 receptor binding domain (RBD) being ≥ 256 World Health Organization (WHO) EU/mL. With initiation of the multicenter component of the trial, neutralization testing was added to the plasma selection process such that selected plasma units showed neutralizing function, defined as a half-maximal neutralization titer ≥ 50 (NT50 ≥ 50) using a replication-competent chimeric vesicular stomatitis virus expressing the SARS-CoV-2 spike protein (e-Methods Section C).15 , 18 In the spring of 2021, a total of 11 units of COVID-19 convalescent plasma units were supplied by Vitalant, a commercial US-based blood bank.
Patients randomized to the convalescent plasma group received an IV infusion of a single unit (200-399 mL) of ABO-compatible COVID-19 convalescent plasma. Patients randomized to receive placebo received a single 250 mL IV infusion of lactated Ringer’s solution with multivitamin additives. The study infusion was delivered as soon as possible and within 24 h of randomization. Use of open-label convalescent plasma in the 14 days following the study infusion was a protocol deviation. Enrollment in other COVID-19 clinical trials, such as those evaluating anti-SARS-CoV-2 monoclonal antibody therapies, was not permitted. Other aspects of clinical management, including the use of remdesivir, corticosteroids, immunomodulators, and oxygen therapy, were performed by the treating clinicians without influence from the study protocol. Concomitant medications were recorded through hospital discharge.
Laboratory Assessments
Plasma donors had serum specimens collected at the time of their donation. These specimens were tested for IgG antibodies against the SARS-CoV-2 RBD and for neutralization of a virus displaying the SARS-CoV-2 spike protein.15 Using these results, convalescent plasma units were characterized in terms of anti-RBD antibody quantification and neutralization titers (e-Methods Section D).
Trial participants had serum specimens collected at baseline (following consent for trial participation and prior to study product infusion) and postinfusion (approximately 24 h after study product infusion). These samples were tested with the same quantitative anti-RBD antibody assay. Patients with a baseline serum specimen with ≤ 3.0 WHO EU/mL on the anti-RBD assay were classified as “seronegative,” indicating no endogenous anti-SARS-CoV-2 antibodies detected prior to the study infusion (e-Methods Section D).
Outcomes
The primary outcome was the patient’s clinical status (disease severity) 14 days following the study infusion on a seven-category ordinal scale (the COVID-19 Clinical Progression Scale). The seven categories were: (1) not hospitalized with resumption of normal pre-illness activities; (2) not hospitalized but unable to resume normal pre-illness activities (including use of home supplemental oxygen by patients who did not use home oxygen pre-illness); (3) hospitalized and not on supplemental oxygen; (4) hospitalized and on standard-flow supplemental oxygen; (5) hospitalized and on high-flow oxygen therapy or noninvasive mechanical ventilation; (6) hospitalized and on invasive mechanical ventilation or extracorporeal membrane oxygenation; and (7) death. While the patient was hospitalized, the ordinal scale category was identified by direct patient observation and medical record review. Following hospital discharge, patients were contacted by telephone to distinguish between category 1 and 2 using questions consistent with validated health status measures.20 , 21 This scale was developed by the WHO as a patient-centered outcome for COVID-19 trials22 and has been used in multiple prior trials.23, 24, 25, 26
The trial included 13 secondary efficacy outcomes, including: all-cause, all-location mortality at 14 and 28 days; clinical status on the COVID-19 Clinical Progression Scale at 2, 7, and 28 days; time to hospital discharge; time to recovery (defined as the earlier of oxygen liberation or hospital discharge); and several types of support-free days through day 28, including hospital-free days, oxygen-free days, ICU-free days, ventilator-free days, and vasopressor-free days. The key safety outcome was clinical evidence of a transfusion reaction within 6 h of initiation of the study infusion. Details of each outcome are described in e-Methods Section E.
Statistical Analysis
The trial was designed and analyzed with a likelihood framework27 , 28 because rapid changes in the COVID-19 pandemic could result in a need to change the interim analysis schedule or sample size. An analysis plan using the likelihood approach retains interpretability when such changes are made.29 , 30
Efficacy outcomes were analyzed in the intention-to-treat population, which comprised all randomized patients who did not withdraw consent, by comparing patients randomized to the convalescent plasma group vs the placebo group, with the placebo group serving as the referent. The primary outcome was analyzed with a multivariable cumulative probability ordinal regression model with logit link adjusted for the following baseline (preinfusion) characteristics: age, sex, Sequential Organ Failure Assessment score, COVID-19 Clinical Progression Scale category, duration of COVID-19 symptoms, and enrolling site (as a random effect). Model output was an adjusted OR (aOR), with an aOR < 1.0 indicating more favorable outcomes on the scale in the convalescent plasma group compared with the placebo group. Uncertainty for the aOR was quantified with a one-seventh support interval (1/7 SI), which can be interpreted similarly to 95% CIs; however, unlike 95% CIs, SIs maintain interpretability if circumstances require changes to the timing or frequency of interim analyses.
The prespecified target sample size was 1,000 participants. Power of the trial with 1,000 participants was estimated via simulations as described in the Statistical Analysis Plan. These simulations showed that while maintaining a type I error rate < 0.05, a sample size of 1,000 patients would provide 80% power to detect an aOR for the primary outcome ≤ 0.73, a difference considered clinically important in prior COVID-19 trials.23 , 25 , 26 The trial had three planned interim analyses after primary outcome data were available for approximately 150, 450, and 750 patients, and had flexibility to add interim analyses based on changes in the pandemic or emerging data on COVID-19 convalescent plasma.
In a secondary analysis, the primary outcome was analyzed in an as-treated population, consisting of patients in the intention-to-treat population who started the assigned study infusion (ie, who received any volume of the assigned convalescent plasma vs placebo solution). Heterogeneity of treatment effect for the primary outcome by baseline characteristics was assessed for the following variables: trial participant’s serum anti-RBD antibody concentration; duration of COVID-19 symptoms; age; race/ethnicity; indicators of illness severity; plasma donor’s serum anti-RBD antibody concentration; and plasma donor’s anti-SARS-CoV-2 neutralization titer.
Secondary efficacy outcomes were analyzed in the intention-to-treat population using multivariable regression models with the same covariates as the model for the primary outcome (e-Methods Section E). Safety outcomes and adverse events were analyzed without covariate adjustment.
The presentation of results included between-group differences with 1/7 SIs. Results with a 1/7 SI that did not cross the null were deemed to reflect a statistical difference. The widths of SIs were not adjusted for multiplicity. Analyses were conducted with R version 4.1.1 (R Foundation for Statistical Computing) and STATA 16.1 (StataCorp).
Halting Enrollment
On June 1, 2021, a fourth interim analysis was completed, at which time 974 (97.4%) patients of the prespecified sample size of 1,000 patients had been enrolled. This interim analysis was called due to increasing COVID-19 vaccination rates in the United States. The Data and Safety Monitoring Board recommended halting further enrollment in the trial based on a probability > 0.999 that the trial would not demonstrate efficacy if it continued to the planned sample size (e-Methods Section F).
Results
Convalescent Plasma Used in the Trial
During the trial, 463 units of COVID-19 convalescent plasma were used, including 452 (97.6%) units originating from trial-specific plasma drives and 11 (2.4%) units purchased from Vitalant. Most units that started the screening process for use in the trial were excluded for not meeting the rigorous selection criteria (e-Fig 1). Median (interquartile range [IQR]) serum anti-RBD IgG concentration for donors of the 463 plasma units used in the trial was 954 (558-2,252) WHO EU/mL (e-Table 2). Among the 463 units, 413 (89.2%) had confirmed neutralizing activity with NT50 ≥ 50; the 50 (10.8%) units without neutralizing activity were collected during the single-center component of the trial and, despite having serum anti-RBD IgG ≥ 256 WHO EU/mL, were retrospectively found to have an NT50 < 50.
Trial Participants
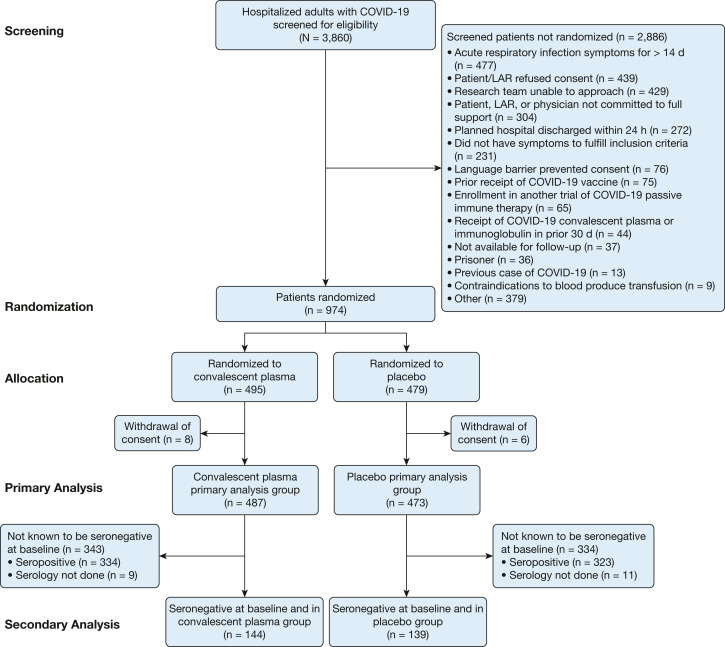
Among 3,860 patients screened for potential trial participation, 974 patients were randomized to treatment (Fig 1 ). After randomization, eight patients in the convalescent plasma group and six patients in the placebo group withdrew consent for trial participation, resulting in a primary analytical population of 960 patients. Median (IQR) age was 60 (49-70) years, 410 (42.7%) were female, 184 (19.2%) were Black, 143 (14.9%) were Hispanic/Latinx, 210 (21.9%) were receiving high-flow oxygen therapy or noninvasive ventilation, and 125 (13.0%) were receiving invasive mechanical ventilation at randomization (e-Tables 3 and 4, Table 1 15 , 22 , 31). Median (IQR) duration of COVID-19 symptoms prior to randomization was 8 (5-10) days. Serum anti-RBD antibody concentrations at baseline ranged from undetectable to 142,564 WHO EU/mL, with a median value of 18 WHO EU/mL; 283 of 940 (30.1%) patients were seronegative (anti-RBD IgG concentration ≤ 3 WHO EU/mL), and 722 of 940 (76.8%) patients had an anti-RBD IgG concentration under the threshold correlated with neutralization (≤ 256 WHO EU/mL). Serum anti-RBD antibody concentration correlated poorly with duration of COVID-19 symptoms (e-Fig 2).
Figure 1.
Flow diagram for enrollment of trial participants. LAR = legally authorized representative.
Table 1.
Baseline Patient Characteristics
| Characteristic | COVID-19 Convalescent Plasma (n = 487) | Placebo (n = 473) |
|---|---|---|
| Age, median (IQR), y | 60 (50-70) | 60 (49-70) |
| Sex, No. (%) | ||
| Female | 206 (42.3) | 204 (43.1) |
| Male | 281 (57.7) | 269 (56.9) |
| Race/ethnicity, No. (%) | ||
| Non-Hispanic White | 272 (55.9) | 285 (60.3) |
| Non-Hispanic Black | 104 (21.4) | 80 (16.9) |
| Hispanic | 72 (14.8) | 71 (15.0) |
| Asian | 14 (2.9) | 11 (2.3) |
| American Indian or Alaska Native | 6 (1.2) | 5 (1.1) |
| Native Hawaiian or other Pacific Islander | 2 (0.4) | 4 (0.8) |
| Multi-race or other | 8 (1.6) | 11 (2.3) |
| Declined to answer | 9 (1.8) | 6 (1.3) |
| Living location prior to onset of COVID-19, No. (%) | ||
| Home in the community without in-house health care | 467 (95.9) | 455 (96.2) |
| Assisted living or nursing home | 16 (3.3) | 13 (2.7) |
| Other | 4 (0.8) | 5 (1.1) |
| BMI, median (IQR), kg/m2 | 32 (28-39) | 32 (28-39) |
| Chronic conditions, No. (%) | ||
| Hypertension | 303/475 (63.8) | 269/470 (57.2) |
| Diabetes mellitus | 176/483 (36.4) | 150/472 (31.8) |
| Chronic lung disease | 125 (25.7) | 135 (28.5) |
| Chronic kidney disease | 90/486 (18.5) | 80 (16.9) |
| Chronic liver disease | 25/486 (5.1) | 19 (4.0) |
| Chronic neurologic disease | 61/486 (12.6) | 56 (11.8) |
| Malignancy | 34/486 (7.0) | 44 (9.3) |
| Location at time of randomization, No. (%) | ||
| ED | 28 (5.7) | 33 (7.0) |
| Hospital ward | 253 (52.0) | 258 (54.5) |
| Step-down or intermediate care unit | 53 (10.9) | 58 (12.3) |
| ICU | 153 (31.4) | 124 (26.2) |
| Symptoms of acute respiratory infection, No. (%) | ||
| Shortness of breath | 420 (86.2) | 407 (86.0) |
| Cough | 366 (75.2) | 344 (72.7) |
| Fever (temperature > 37.5°C) | 244 (50.1) | 260 (55.0) |
| Duration of COVID-19 symptoms prior to randomization, median (IQR), d | 8 (5-10) | 8 (5-10) |
| Time between hospital presentationa and randomization, median (IQR), h | 40 (25-65) | 42 (25-65) |
| COVID-19 Clinical Progression Scale category at randomization, No. (%)b | ||
| 3: hospitalized and not on supplemental oxygen | 44 (9.0) | 44 (9.3) |
| 4: hospitalized and on standard-flow oxygen | 268 (55.0) | 269 (56.9) |
| 5: hospitalized and on nasal high-flow oxygen therapy or noninvasive ventilation | 112 (23.0) | 98 (20.7) |
| 6: hospitalized and on invasive mechanical ventilation or ECMO | 63 (12.9) | 62 (13.1) |
| Medication use at time of randomization, No. (%) | ||
| Remdesivir | 343 (70.4) | 337 (71.2) |
| Corticosteroids | 429/485 (88.5) | 401/472 (85.0) |
| Vasopressors | 45 (9.2) | 42/472 (8.9) |
| Total SOFA score at randomization, median (IQR)c | 3 (2-5) | 3 (2-4) |
| Blood type, No. (%) | ||
| A | 170/486 (35.0) | 183 (38.7) |
| B | 63/486 (13.0) | 45 (9.5) |
| AB | 21/486 (4.3) | 13 (2.7) |
| O | 232/486 (47.7) | 232 (49.0) |
| Serum anti-SARS-CoV-2 receptor binding domain antibody status at randomization, No. (%)d | ||
| Not measured | 9 (1.8) | 11 (2.3) |
| Measured | 478 (98.2) | 462 (97.7) |
| Antibody concentration, median (IQR), WHO EU/mL | 19 (2-248) | 17 (2-166) |
| By threshold for seropositivity (3 WHO EU/mL), No. (%) | ||
| Seronegative (≤ 3 WHO EU/mL) | 144/478 (30.1) | 139/462 (30.1) |
| Seropositive (> 3 WHO EU/mL) | 334/478 (69.9) | 323/462 (69.9) |
| By threshold correlated with neutralizing function (256 WHO EU/mL), No. (%) | ||
| ≤ 256 WHO EU/mL | 360/478 (75.3) | 362/462 (78.4) |
| > 256 WHO EU/mL | 118/478 (24.7) | 100/462 (21.6) |
ECMO = extracorporeal membrane oxygenation; IQR = interquartile range; SOFA = Sequential Organ Failure Assessment; WHO = World Health Organization.
Time of hospital presentation was defined as the time of the first contact with an acute care hospital during the health-care episode that resulted in the hospitalization during which the patient was enrolled. For patients who initially presented to the ED, time of hospital presentation was the time of ED arrival. For patients directly hospitalized without presenting to the ED, time of hospital presentation was the time of arrival at the admission unit.
The COVID-19 Clinical Progression Scale is a seven-category ordinal scale that classifies a patient’s clinical status.22 Higher scores indicate more severely ill clinical status. Patients in the following categories at screening were not eligible for enrollment: (1) not hospitalized with resumption of normal pre-illness activities; (2) not hospitalized but unable to resume normal pre-illness activities; and (7) dead. Patients in categories 3 through 6 at randomization were eligible for the trial; the distribution of categories at randomization is displayed in the table.
SOFA score31 categorizes illness severity based on organ dysfunction across six organ systems: respiratory, coagulation, liver, cardiovascular, CNS, and renal. SOFA scores range from 0 to 24, with higher scores indicating greater illness severity. A SOFA score of 2 indicates moderate dysfunction in one organ system or mild dysfunction in two organ systems.
Trial patients had serum collected prior to study infusion. These baseline serum samples underwent quantitative measurement for antibodies against the SARS-CoV-2 spike protein receptor binding domain (RBD). Patients with an anti-RBD antibody concentration ≤ 3 WHO EU/mL were classified as seronegative to indicate no detectable endogenous antibodies. Patients with an anti-RBD concentration > 3 WHO EU/mL were classified as seropositive. Based on prior work,15 an anti-RBD antibody concentration > 256 WHO EU/mL correlated with neutralizing antibody function against SARS-CoV-2. Hence, patients with an anti-RBD antibody concentration ≤ 256 WHO EU/mL were classified as having an antibody concentration correlated with no detectable neutralizing function.
Among 960 patients in the primary analytical population, 487 (50.7%) were randomized to receive convalescent plasma and 473 (49.3%) to placebo. Successful follow-up for survival through day 28 was completed for 947 (98.6%) patients; five patients in the convalescent plasma group and eight patients in the placebo group were lost to follow-up for 28-day survival.
Study Infusions and Co-interventions
Among 960 patients in the primary analytical population, 928 (96.7%) patients had the study infusion initiated (e-Table 5). Among 870 patients with anti-RBD IgG measured approximately 24 h after study infusion, median (IQR) anti-RBD serum IgG concentration was 148 (22 to 741) WHO EU/mL in the convalescent plasma group and 93 (4 to 617) WHO EU/mL in the placebo group (e-Table 6). In the samples collected 24 h following study infusion, 43 of 446 (9.6%) patients in the convalescent plasma group and 113 of 438 (25.8%) in the placebo group had anti-RBD concentrations < 3 WHO EU/mL (seronegative threshold). During the same hospitalization as trial enrollment, 763 (79.5%) patients received remdesivir and 858 (89.4%) received corticosteroids (e-Table 7).
Primary Outcome
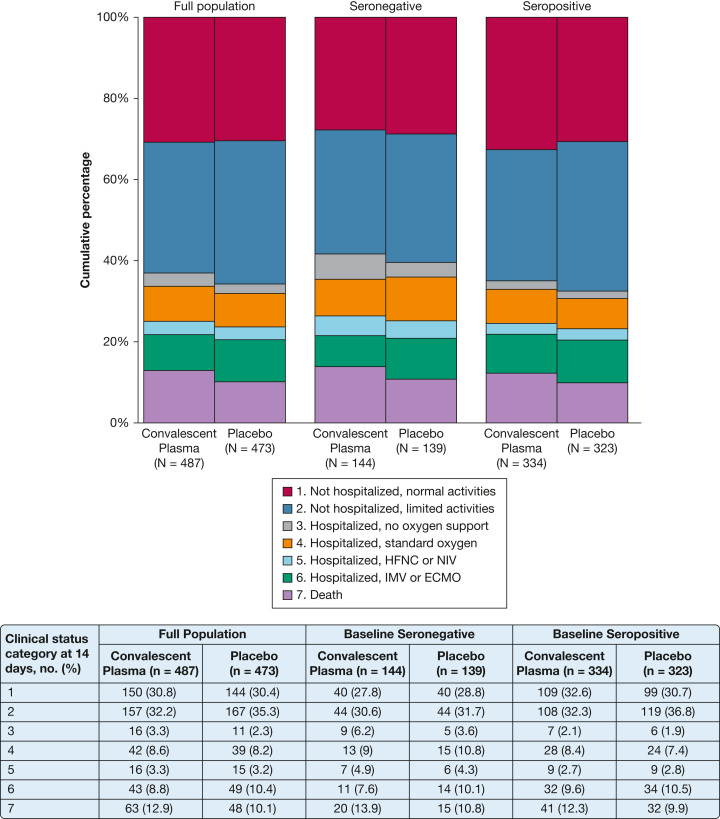
At 14 days following study infusion, there was no difference in the COVID-19 Clinical Progression Score between the convalescent plasma group and the placebo group in the overall population (aOR, 1.04; 1/7 SI, 0.82-1.33), the seronegative population (aOR, 1.15; 1/7 SI, 0.74-1.80), or the seropositive population (aOR, 0.96; 1/7 SI, 0.72-1.30) (Fig 2 , Table 2 ). There was no difference in the primary outcome when the analysis was limited to the as-treated population, when patients in the convalescent plasma group who received units without neutralizing function were excluded (post hoc analysis), or when the analysis was stratified into subgroups defined according to the patient’s age, race/ethnicity, or indicators of illness severity (e-Fig 3, e-Table 8). Heterogeneity of treatment effect for the primary outcome was not identified based on the infused plasma’s anti-RBD IgG concentration or neutralization titer (e-Fig 4), the patient’s baseline serum anti-RBD IgG concentration (e-Fig 5), or the patient’s duration of COVID-19 symptoms (e-Fig 6).
Figure 2.
Clinical Status on the COVID-19 Clinical Progression Scale 14 days after study infusion by treatment group (COVID-19 convalescent plasma vs placebo) for the full trial population (N = 960), seronegative population at baseline (serum anti-receptor binding domain [RBD] IgG concentration ≤ 3 World Health Organization EU/mL prior to study infusion; n = 283), and the seropositive population at baseline (serum anti-RBD IgG concentration > 3 World Health Organization EU/mL prior to study infusion; n = 657). The seven categories of the COVID-19 Clinical Progression Scale were: (1) not hospitalized with resumption of normal pre-illness activities; (2) not hospitalized but unable to resume normal pre-illness activities; (3) hospitalized and not on supplemental oxygen; (4) hospitalized and on standard-flow supplemental oxygen; (5) hospitalized and on HFNC oxygen therapy or NIV; (6) hospitalized and on IMV or ECMO; and (7) death. The percentage of patients in each of the seven categories at day 14 is displayed in the figure and accompanying table according to trial group assignment. There was no difference between the distribution of clinical status categories between the convalescent plasma group and the placebo group for the full trial population (adjusted OR [aOR], 1.04; one-seventh SI [1/7 SI], 0.82-1.33), seronegative population (aOR, 1.15; 1/7 SI, 0.74-1.80), or seropositive population (aOR, 0.96; 1/7 SI, 0.72-1.30). ECMO = extracorporeal membrane oxygenation; HFNC = high-flow nasal cannula; IMV = invasive mechanical ventilation; NIV = noninvasive mechanical ventilation.
Table 2.
Outcomes and Adverse Events
| Outcome | COVID-19 Convalescent Plasma (n = 487) | Placebo (n = 473) | aOR, OR, or Adjusted Hazard Ratio (1/7 SI)a | Unadjusted Difference In Percentage Points (1/7 SI) |
|---|---|---|---|---|
| Primary efficacy outcome | ||||
| COVID Clinical Progression Ordinal Scale at 14 d, median (IQR)b | 2 (1-5) | 2 (1-4) | 1.04 (0.82 to 1.33) | |
| Secondary efficacy outcomes | ||||
| All-cause, all-location death, No. (%) | ||||
| At 14 d | 63/482 (13.1) | 48/465 (10.3) | 1.30 (0.79 to 2.12) | 2.7 (–1.4 to 6.9) |
| At 28 d | 89/482 (18.5) | 80/465 (17.2) | 1.04 (0.69 to 1.58) | 1.3 (–3.6 to 6.2) |
| Time to death through 28 d | 1.21 (0.88 to 1.64) | |||
| Time to hospital discharge | 1.00 (0.86 to 1.16) | |||
| Time to recovery (earlier of final receipt of oxygen or hospital discharge) | 1.00 (0.88 to 1.14) | |||
| COVID Clinical Progression Scale, median (IQR)b | ||||
| At 2 d | 4 (4-5) | 4 (4-5) | 1.26 (0.98 to 1.63) | |
| At 7 d | 3 (2-5) | 3 (2-5) | 1.12 (0.88 to 1.41) | |
| At 28 d | 2 (1-4) | 2 (1-4) | 1.19 (0.92 to 1.53) | |
| Support-free days through day 28, median (IQR) | ||||
| Hospital-free days | 20 (0-24) | 21 (0-24) | 0.98 (0.77 to 1.24) | |
| Oxygen-free days | 21 (0-25) | 21 (0-25) | 0.95 (0.75 to 1.21) | |
| ICU-free days | 28 (2-28) | 28 (13-28) | 0.92 (0.68 to 1.23) | |
| Ventilator-free days | 28 (18-28) | 28 (22-28) | 0.87 (0.61 to 1.24) | |
| Vasopressor-free days | 28 (27-28) | 28 (26-28) | 1.11 (0.78 to 1.60) | |
| Safety outcomes and adverse events, No. (%) | ||||
| Transfusion reaction | 6 (1.2) | 0 (0) | 1.2 (0.4 to 2.5) | |
| ≥ 1 Adverse event | 47 (9.7) | 33 (7.0) | 1.42 (0.86 to 2.27) | 2.7 (–0.8 to 6.2) |
| ≥ 1 Serious adverse event | 21 (4.3) | 16 (3.4) | 1.29 (0.66 to 2.51) | 0.9 (–1.5 to 3.4) |
1/7 SI = one-seventh support interval; aOR = adjusted OR; IQR = interquartile range.
Models for the primary and secondary efficacy outcomes were constructed with trial group assignment (COVID-19 convalescent plasma vs placebo) as the independent variable, the outcome as the dependent variable, and the following co-variates: age (as a restricted cubic spline with three knots), sex, Sequential Organ Failure Assessment score, baseline COVID-19 Clinical Progression Scale category (as a second degree polynomial), duration of COVID-19 symptoms (as a restricted cubic spline with three knots), and enrolling site (as a random effect). Multivariable cumulative probability ordinal regression models with logit link were used to analyze ordinal outcomes (COVID-19 Clinical Progression Scale outcomes and support-free outcomes). Multivariable logistic regression models were used for binary outcomes (binary death outcomes). Multivariable proportional hazards (Cox) models were used for time to event outcomes (time to death through 28 days, time to hospital discharge, and time to recovery). Discharge and recovery were potentially censored by death. Logistic regression models without covariate adjustment were used for safety outcomes and adverse events. ORs < 1.0 indicated more favorable outcomes for patients in the convalescent plasma group compared with the placebo group for the following outcomes: COVID Clinical Progression Scale (aOR < 1.0 indicated lower score on the scale); death (aOR < 1.0 indicated fewer deaths); transfusion reaction (aOR < 1.0 indicated fewer transfusion reactions); and adverse events (aOR < 1.0 indicated fewer adverse events). ORs > 1.0 indicate more favorable outcomes for patients in the convalescent plasma group compared with the placebo group for the support-free outcomes (aOR > 1.0 indicated more support free days). Variability for each OR was represented by a 1/7 SI.
The COVID Clinical Progression Scale is a seven-category ordinal scale that classifies a patient’s clinical status.22 The seven categories are: (1) not hospitalized with resumption of normal pre-illness activities; (2) not hospitalized but unable to resume normal pre-illness activities (including use of home supplemental oxygen by patients who did not use home oxygen pre-illness); (3) hospitalized and not on supplemental oxygen; (4) hospitalized and on standard flow supplemental oxygen; (5) hospitalized and on nasal high-flow oxygen therapy or noninvasive mechanical ventilation; (6) hospitalized and on invasive mechanical ventilation or extracorporeal membrane oxygenation; and (7) death.
Mortality and Other Secondary Outcomes
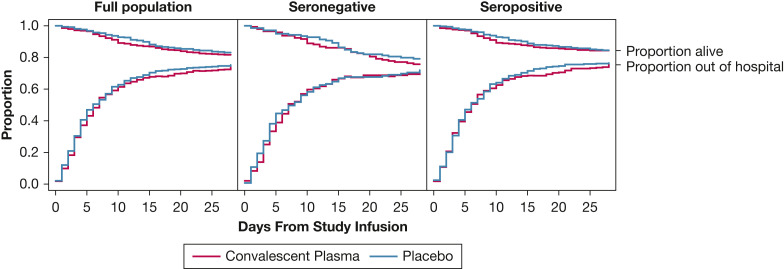
At 28 days following study infusion, 89 of 482 (18.5%) patients in the convalescent plasma group and 80 of 465 (17.2%) patients in the placebo group had died (aOR, 1.04; 1/7 SI, 0.69-1.58) (Fig 3 ). In the seronegative population, 28-day mortality was not different between the convalescent plasma group (35 of 143 [24.5%]) and the placebo group (29 of 136 [21.3%]) (aOR, 1.12; 1/7 SI, 0.56-2.23). In the seropositive population, 28-day mortality was not different between the convalescent plasma group (52 of 330 [15.8%]) and the placebo group (50 of 319 [15.7%]) (aOR, 1.00; 1/7 SI, 0.58-1.72). No heterogeneity of treatment effect was identified for 28-day mortality (e-Figs 7-9).
Figure 3.
Survival and hospital discharge through 28 days after study infusion by treatment group assignment (COVID-19 convalescent plasma vs placebo) for the full trial population (N = 960), the seronegative population at baseline (serum anti-receptor binding domain [RBD] IgG concentration ≤ 3 World Health Organization EU/mL prior to study infusion; n = 283), and the seropositive population at baseline (serum anti-RBD IgG concentration > 3 World Health Organization EU/mL prior to study infusion; n = 657). In each plot, the convalescent plasma group is represented by blue lines and the placebo group by red lines. The top set of lines are Kaplan-Meier survival plots. The bottom set of lines denote the proportion of participants alive and discharged from the hospital. Patient disposition is represented by the three locations within the plot area: dead, represented by the area above the survival lines; alive and still in the hospital, represented by the area between the survival and discharge lines; and discharged from the hospital alive, represented by the area under the discharge lines. The proportion in each disposition state is denoted by the relative height of the region for each day. On study day 1, the vast majority of participants were alive and in the hospital (middle region). Over time, the proportion of participants in the alive and discharged state (lower region) and dead state (upper region) increases, which gives rise to the “funnel” shape of the plot. Participants could move from either in-hospital or discharged states to the dead state. Patients were followed up via medical records and telephone follow-up until 28 days following study infusion. Patients lost to follow-up were included in the risk-set for the portion of days for which disposition was known. A patient was considered discharged from the hospital once discharged from the index hospitalization; re-hospitalizations were not considered in this analysis. In model-based estimates of treatment effect, there was no difference in time-to-death through 28 days between the convalescent plasma group and the placebo group for the full trial population (adjusted hazard ratio [aHR], 1.20; one-seventh SI [1/7 SI], 0.88-1.64), the seronegative population (aHR, 1.19; 1/7 SI, 0.71-1.99), or the seropositive population (aHR: 1.17; 1/7 SI, 0.78-1.76). Furthermore, there was no significant difference in a model of time to hospital discharge through 28 days between the convalescent plasma group and the placebo group in the full trial population (aHR, 1.00; 1/7 SI, 0.86-1.16), the seronegative population (aHR, 0.94; 1/7 SI, 0.71-1.25), or the seropositive population (aHR, 1.05; 1/7 SI, 0.88-1.26).
There were no differences in any of the 13 secondary efficacy outcomes between the convalescent plasma and placebo groups in the overall population (e-Table 9, Table 2), the seronegative population (e-Table 10), or the seropositive population (e-Table 11).
Safety Outcomes and Adverse Events
A transfusion reaction was reported in six of 487 (1.2%) patients in the convalescent plasma group and 0 of 473 (0%) patients in the placebo group (e-Table 12). A total of 27 serious adverse events were reported from 21 of 487 (4.3%) unique patients in the convalescent plasma group and 18 serious adverse events from 16 of 473 (3.4%) unique patients in the placebo group (e-Table 13).
Discussion
In this multicenter, randomized, placebo-controlled clinical trial among adults hospitalized with COVID-19 in the United States, treatment with COVID-19 convalescent plasma showed no signs of clinical efficacy. Point estimates for both the clinical status ordinal scale outcome and mortality all favored placebo over convalescent plasma for both the full population and the subgroup of patients seronegative for endogenous anti-SARS-CoV-2 antibodies prior to study infusion.
This trial included several strengths that add to the literature on COVID-19 convalescent plasma and address some of the critiques of prior convalescent plasma trials.8, 9, 10 , 13 , 14 The trial design included a placebo group, which received a crystalloid solution rather than plasma without anti-SARS-CoV-2 antibodies, and blinded study procedures to optimize internal validity. Furthermore, convalescent plasma used in the trial was selected based on the presence of anti-SARS-CoV-2 antibodies with neutralizing activity, whereas most prior trials used convalescent plasma selected based on binding antibody titers only without laboratory confirmation of neutralizing activity.8, 9, 10 , 14 Laboratory confirmation of neutralizing activity is an important step if the goal is to ensure every convalescent plasma unit has neutralizing function because some convalescent plasma with high binding titers does not have neutralizing function.15 Unlike some prior observational studies,6 and similar to prior randomized trials,14 results of the current trial did not suggest that convalescent plasma with higher antibody concentrations (within the range of concentrations used in this trial) was more likely to be beneficial. Null results in this trial, including no evidence of heterogeneity of treatment effect by neutralization activity in the transfused plasma units, suggest that treatment even with the most carefully selected plasma with neutralizing function is unlikely to improve clinical outcomes.
This trial also characterized patients’ endogenous anti-SARS-CoV-2 antibody status prior to study infusion. Approximately one-third of the trial population had no endogenous anti-SARS-CoV-2 antibodies at baseline (< 3.0 WHO EU/mL) and approximately three-quarters of the population had endogenous anti-SARS-CoV-2 antibodies below the concentration correlated with neutralizing function (< 256 WHO EU/mL); treatment with convalescent plasma did not show any signs of clinical efficacy in populations limited to patients with no or low levels of endogenous anti-SARS-CoV-2 antibodies. This finding suggests that treatment with convalescent plasma is unlikely to be efficacious even with high-quality neutralizing convalescent plasma among patients with a limited endogenous immune response against SARS-CoV-2.
Despite initial promising signals from observational studies evaluating convalescent plasma as a treatment for severely ill, hospitalized patients with COVID-19,6 a series of randomized clinical trials have now consistently demonstrated null findings.10 , 13 , 14 Compared with many anti-SARS-CoV-2 monoclonal antibody therapies,16 , 17 , 24 the convalescent plasma used in this trial delivered a relatively small dose of neutralizing antibodies; it is possible that other passive immunity therapies that deliver significantly higher doses of neutralizing antibodies could demonstrate efficacy.
The current study had certain limitations. First, prior vaccination against COVID-19 was an exclusion for both plasma donation and trial participation. Trial results may not be directly generalizable to vaccinated populations. Second, the volume of convalescent plasma used in this trial was one unit (200-399 mL); some prior trials and clinical protocols used larger volumes of convalescent plasma. Third, the convalescent plasma used in this trial was mostly collected from donors in Tennessee, while recipients were geographically dispersed across the United States. Some have theorized that collecting convalescent plasma from people in geographic proximity to those who will receive it could increase efficacy.32 Fourth, this trial started as a single-center trial before expanding to a multicenter national trial; participants enrolled in the single-center component of the trial were included in the overall analysis. Methods for selecting convalescent plasma units were optimized during the early, single-center trial period and then standardized for the multicenter component of the trial. During the single-center component of the trial, 50 participants (representing 10.3% of participants randomized to the convalescent plasma group) were treated with plasma units that did not exhibit neutralizing function above the threshold ultimately chosen to select plasma for the remainder of the trial. A post hoc sensitivity analysis that excluded these patients showed no substantive differences in trial results. Fifth, median duration of COVID-19 symptoms prior to randomization in this trial was 8 days. Although analyses evaluating for heterogeneity of treatment effect did not suggest that patients with shorter duration of symptoms benefited more from convalescent plasma than those with longer duration of symptoms, this trial did not rule out the possibility of benefit for patients treated shortly following symptom onset. Sixth, SARS-CoV-2 variants were not determined in this trial; enrollment occurred during periods of predominant circulation by the original wild-type virus and B.1.1.7 (Alpha) variant in the United States.
Interpretation
Among adults hospitalized with COVID-19, treatment with neutralizing COVID-19 convalescent plasma did not lead to improvement in clinical status at day 14. These results do not support the use of convalescent plasma as a treatment for adults hospitalized with COVID-19.
Acknowledgments
Author contributions: W. H. S. takes responsibility for this work overall. W. H. S. and T. W. R. led execution of the clinical trial. Data analyses were conducted at Vanderbilt University Medical Center by T. G. S. and L. W., who had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Further details of author contributions are as follows: study concept and design, W. H. S., A. P. W., T. G. S., R. H. C., J. D. Chappell, J. E. C., P. G., I. T., C. J. L., J. M. P. J. M. P., J. P. R., G. R. B., and T. W. R.; acquisition of data, W. H. S., A. P. W., H. S., J. M., T. G. S., V. D. C., H. R. O., N. I. S., C. H., A. A. G., L. C., N. J. J., D. J. H., S. J. J., M. J. M., E. S. H., S. R. P., M. L.-V., W. E. A., M. d. W., D. H., C. S. C., C. M., C. S., A. E. J., J. S. R., S. M., T. S. I., X. Q., S. J. S., A. S., R. D. F., A. J. B.-H., J. D. Casey, J. M. P., J. P. R., G. R. B., and T. W. R.; laboratory analyses, R. H. C., J. D. Chappell, J. E. C., M. R. D., P. G., L. J. S., R. E. S., I. T., S. M. Y., and E. J. B.; statistical analysis, T. G. S., C. J. L., and L. W.; and drafting of the first version of the manuscript, W. H. S. All authors contributed to interpretation of data and critical revision of the manuscript for important intellectual content.
Funding/support: Primary funding for this work was provided by NCATS [3UL1TR002243-04S3]. Funding for pilot work was provided by the Dolly Parton COVID-19 Research Fund. Funding for Research Electronic Data Capture, trial innovation work, and the other data tools was provided by NCATS [UL1TR002243].
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. H. C. reports grants from IDBiologics and Takeda Vaccines, grants and other from AstraZeneca, outside the submitted work; and has patents for COVID-19 human monoclonal antibodies (multiple) with licenses to AstraZeneca, IDBiologics, and Leinco and royalties paid to the US government. J. D. Casey reports grants from the National Institute of Health (NIH) outside the submitted work. J. D. Chappell reports grants from the NIH and grants from the Dolly Parton COVID-19 Research Fund during the conduct of the study. J. E. C. reports serving as a consultant for Luna Innovations, Merck, and GlaxoSmithKline, and as a member of the Scientific Advisory Board of Meissa Vaccines and being the founder of IDBiologics; the laboratory of J. E. C. received sponsored research agreements from AstraZeneca, Takeda, and IDBiologics during the conduct of the study. Vanderbilt University has applied for patents for some of the antibodies in this paper, for which J. E. C. is an inventor. W. E. A. reports grants from NCATS, during the conduct of the study, and grants from Biomedical Advanced Research and Development Authority (BARDA) outside the submitted work. P. G. reports that he is a co-inventor with Vanderbilt University on patents for SARS-CoV-2 human monoclonal antibodies (multiple) with licenses to AstraZeneca, IDBiologics, and Leinco and the US Government has certain rights to these antibodies. M. R. D. reports grants from the NIH and the Dolly Parton COVID-19 Research Fund during the conduct of this study. A. A. G. reports grants from the NIH, Department of Defense, the Centers for Disease Control and Prevention, and AbbVie, and grants from Faron Pharmaceuticals outside the submitted work. D. J. H. reports grants and personal fees from Cytovale, personal fees from Beckman-Coulter, and grants and personal fees from Opticyte outside the submitted work. C. J. L. reports grants from the NIH, Department of Defense, and the Centers for Disease Control and Prevention outside the submitted work; research contracts from bioMérieux, AbbVie, Entegrion, and Endpoint Health outside the submitted work; stock options in Bioscape Digital outside the submitted work; and patents (not licensed) for risk stratification in sepsis and septic shock issued to Cincinnati Children’s Hospital Medical Center outside the submitted work. T. W. R. reports receiving grant funding from the NIH for conducting this study and personal fees from Cumberland Pharmaceuticals, Inc; personal fees from Sanofi, Inc; and personal fees from Cytovale, Inc outside the submitted work. W. H. S. reports receiving grant funding from the NIH for conducting this study. L. J. S. reports grants from the NIH and grants from Dolly Parton COVID-19 Research Fund during the conduct of the study. None declared (A. P. W., T. G. S., H. S., J. M., C. B. T., V. D. C., H. R. O., N. I. S., C. H.; L. C., N. J. J., S. J. J., M. J. M., E. S. H., S. R. P., M. L.-V., M. d. W., D. H., C. S. C., C. M., C. S., A. E. J., J. S. R., S. M., T. S. I., X. Q., S. J. S., A. S., R. D. F., E. J. B., R. E. S.; I. T., S. M. Y., A. J. B.-H., L. W., J. M. P., J. P. R., G. R. B.).
Role of the sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Data-sharing statement: Data will be available approximately 90 days following publication of the primary manuscript reporting trial results. Deidentified, patient-level data with a supporting data dictionary will be available. Data from this trial are intended to be shared via the National Heart, Lung, and Blood Institute BioData Catalyst, which can be accessed at: https://biodatacatalyst.nhlbi.nih.gov/.
Other contributions: The authors thank all the plasma donors who gave their time and plasma for infusion in the trial. They also thank all the patients who participated in this study. For use of the WHO anti-SARS-COV-2 antibody standard, they thank the anonymous donors of the plasma samples for their consent, which has allowed WHO International standard for anti-SARS-CoV-2 human immunoglobulin to be prepared. The authors also express our gratitude to those who have coordinated the collection of the convalescent plasma: Malcom Semple (University of Liverpool), Lance Turtle (University of Liverpool), Peter Openshaw (Imperial College London), and Kenneth Baillie (University of Edinburgh) on behalf of the ISARIC4C Investigators; and Heli Harvala Simmonds and David Roberts (National Health Service Blood and Transplant). The authors also thank National Institute for Biological Standards and Control Standards Production and Development staff for the formulation and distribution of materials.
Additional information: The e-Figures, e-Sections, and e-Tables are available online under “Supplementary Data.”
Supplementary Data
References
- 1.Johns Hopkins University COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2021. https://coronavirus.jhu.edu/map.html
- 2.World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. 2021. https://covid19.who.int/ [PubMed]
- 3.Roback J.D., Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020;323(16):1561–1562. doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 4.Casadevall A., Pirofski L.-A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham J. Passive antibody therapy in COVID-19. Nat Rev Immunol. 2020;20(7):401–403. doi: 10.1038/s41577-020-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joyner M.J., Carter R.E., Senefeld J.W., et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384(11):1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention and Infectious Diseases Society of America COVID-19 Real-Time Learning Network: Convalescent Plasma. 2021. https://www.idsociety.org/covid-19-real-time-learning-network/therapeutics-and-interventions/convalescent-plasma/
- 8.Agarwal A., Mukherjee A., Kumar G., et al. Convalescent plasma in the management of moderate Covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonovich V.A., Burgos Pratx L.D., Scibona P., et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RECOVERY Collaborative Group Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397(10289):2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janiaud P., Axfors C., Schmitt A.M., et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185–1195. doi: 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korley F.K., Durkalski-Mauldin V., Yeatts S.D., et al. Early convalescent plasma for high-risk outpatients with Covid-19. N Engl J Med. 2021;385(21):1951–1960. doi: 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bégin P., Callum J., Jamula E., et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med. 2021;27(11):2012–2024. doi: 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Writing Committee for the REMAP-CAP Investigators. Estcourt L.J., Turgeon A.F., et al. Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2021;326(17):1690–1702. doi: 10.1001/jama.2021.18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilchuk P., Thomsen I., Yoder S., et al. Standardized two-step testing of antibody activity in COVID-19 convalescent plasma. iScience. 2022;25(1) doi: 10.1016/j.isci.2021.103602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2021;22(5):622–635. doi: 10.1016/S1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ACTIV-3/TICO Bamlanivimab Study Group. Lundgren J.D., Grund B., et al. Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels: a randomized controlled trial. Ann Intern Med. 2022;175(2):234–243. doi: 10.7326/M21-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Self W.H., Stewart T.G., Wheeler A.P., et al. Passive Immunity Trial for Our Nation (PassITON): study protocol for a randomized placebo-control clinical trial evaluating COVID-19 convalescent plasma in hospitalized adults. Trials. 2021;22(1):221. doi: 10.1186/s13063-021-05171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Informatics. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EuroQol Group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 21.Herdman M., Gudex C., Lloyd A., et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO). WHO R&D Blueprint-Novel Coronavirus, COVID-19 Therapeutic Trial Synopsis. 2020. Accessed July 5, 2021. WHO R&D Blueprint-Novel Coronavirus, COVID-19 Therapeutic Trial Synopsis. https://www.who.int/teams/blueprint/covid-19
- 23.Self W.H., Semler M.W., Leither L.M., et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(21):2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ACTIV-3/TICO LY-CoV555 Study Group. Lundgren J.D., Grund B., et al. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021;384(10):905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinner C.D., Gottlieb R.L., Criner G.J., et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wald A. Sequential tests of statistical hypotheses. Ann Math Statist. 1945;16(2):117–186. [Google Scholar]
- 28.Wang S.-J., Blume J.D. An evidential approach to non-inferiority clinical trials. Pharmaceut Statist. 2011;10(5):440–447. doi: 10.1002/pst.513. [DOI] [PubMed] [Google Scholar]
- 29.Royall R. Chapman and Hall; London, England: 1997. Statistical Evidence: A Likelihood Paradigm. [Google Scholar]
- 30.Blume J.D. Likelihood methods for measuring statistical evidence. Statist Med. 2002;21(17):2563–2599. doi: 10.1002/sim.1216. [DOI] [PubMed] [Google Scholar]
- 31.Singer M., Deutschman C.S., Seymour C.W., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunze K.L., Johnson P.W., van Helmond N., et al. Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors. Infectious Diseases (except HIV/AIDS); 2021. http://medrxiv.org/lookup/doi/10.1101/2021.03.19.21253975 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.