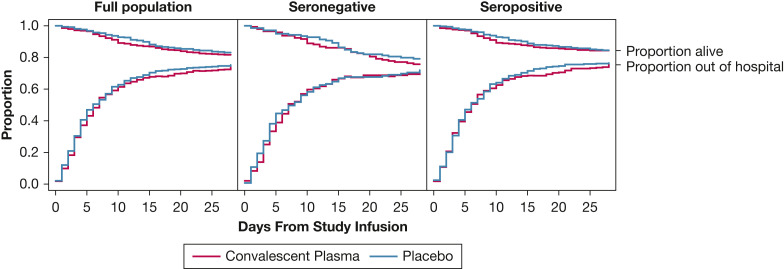
Figure 3.
Survival and hospital discharge through 28 days after study infusion by treatment group assignment (COVID-19 convalescent plasma vs placebo) for the full trial population (N = 960), the seronegative population at baseline (serum anti-receptor binding domain [RBD] IgG concentration ≤ 3 World Health Organization EU/mL prior to study infusion; n = 283), and the seropositive population at baseline (serum anti-RBD IgG concentration > 3 World Health Organization EU/mL prior to study infusion; n = 657). In each plot, the convalescent plasma group is represented by blue lines and the placebo group by red lines. The top set of lines are Kaplan-Meier survival plots. The bottom set of lines denote the proportion of participants alive and discharged from the hospital. Patient disposition is represented by the three locations within the plot area: dead, represented by the area above the survival lines; alive and still in the hospital, represented by the area between the survival and discharge lines; and discharged from the hospital alive, represented by the area under the discharge lines. The proportion in each disposition state is denoted by the relative height of the region for each day. On study day 1, the vast majority of participants were alive and in the hospital (middle region). Over time, the proportion of participants in the alive and discharged state (lower region) and dead state (upper region) increases, which gives rise to the “funnel” shape of the plot. Participants could move from either in-hospital or discharged states to the dead state. Patients were followed up via medical records and telephone follow-up until 28 days following study infusion. Patients lost to follow-up were included in the risk-set for the portion of days for which disposition was known. A patient was considered discharged from the hospital once discharged from the index hospitalization; re-hospitalizations were not considered in this analysis. In model-based estimates of treatment effect, there was no difference in time-to-death through 28 days between the convalescent plasma group and the placebo group for the full trial population (adjusted hazard ratio [aHR], 1.20; one-seventh SI [1/7 SI], 0.88-1.64), the seronegative population (aHR, 1.19; 1/7 SI, 0.71-1.99), or the seropositive population (aHR: 1.17; 1/7 SI, 0.78-1.76). Furthermore, there was no significant difference in a model of time to hospital discharge through 28 days between the convalescent plasma group and the placebo group in the full trial population (aHR, 1.00; 1/7 SI, 0.86-1.16), the seronegative population (aHR, 0.94; 1/7 SI, 0.71-1.25), or the seropositive population (aHR, 1.05; 1/7 SI, 0.88-1.26).