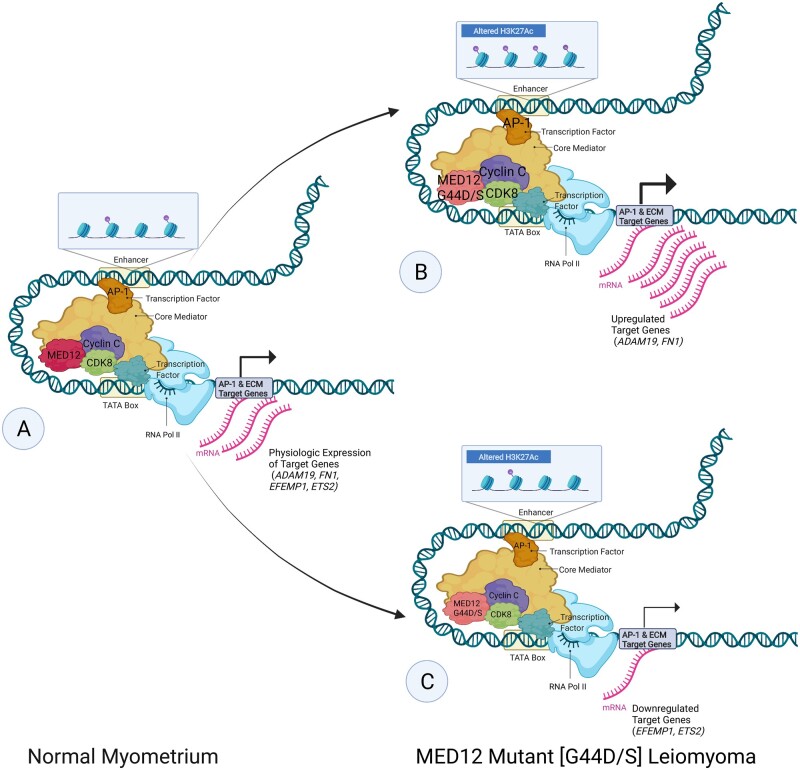
Figure 2.
Altered H3K27Ac and enhancer architecture in MED12 mutant [G44D/S] leiomyoma. Schematic of the proposed (Moyo et al., 2020) differential activator protein 1 [AP-1] and cyclin-dependent kinase 8 [CDK8]/mediator complex subunit 12 [MED12] occupancy, altered enhancer architecture and altered acetylation of the 27th lysine of histone H3 [H3K27Ac] involved in gene dysregulation of AP-1 and extracellular matrix [ECM] target genes in the pathogenesis of G44D/S-mutated MED12 uterine leiomyoma [LM]. Font size represents the strength of recruitment of factors (MED12, CDK8, Cyclin C, AP-1) and arrow thickness represents transcriptional output. Compared to normal myometrium (A), in LM, a subset of genes (ADAM19 indicates ADAM metallopeptidase domain 19; FN1, fibronectin 1) shows higher CDK8/MED12 binding, increased H3K27ac signals found at the enhancer region and enriched AP-1 binding activity. The resulting stronger enhancer–promoter contact found in MED12 mutant [G44D/S] LM leads to the activation of LM-specific gene program (B). Another subset of genes (EFEMP1 indicates EGF containing fibulin extracellular matrix protein 1; ETS2, ETS proto-oncogene 2, transcription factor) in LM shows lower CDK8/MED12 binding, decreased H3K27ac signals found at the enhancer region and depleted AP-1 binding activity. The resulting weaker enhancer–promoter contact found in MED12 mutant [G44D/S] LM leads to the repression of LM-specific gene program (C). Adapted from ‘Eukaryotic Gene Regulation—Transcriptional Initiation’, by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates