Abstract
Objective
To evaluate the association between having concomitant chronic obstructive pulmonary disease (COPD) or asthma, and in-patient mortality and post-discharge management among patients hospitalised for acute heart failure (HF).
Setting
Data were obtained from patients enrolled in the National Heart Failure Audit.
Participants
217 329 patients hospitalised for HF in England–Wales between March 2012 and 2018.
Outcomes
In-hospital mortality, referrals to cardiology follow-up and prescriptions for HF medications were compared between patients with comorbid COPD (COPD-HF) or asthma (asthma-HF) versus HF-alone using mixed-effects logistic regression.
Results
Patients with COPD-HF were more likely to die during hospitalisation, and those with asthma-HF had a reduced likelihood of death, compared with patients who had HF-alone ((adjusted)ORadj, 95% CI: 1.10, 1.06 to 1.14 and ORadj, 95% CI: 0.84, 0.79 to 0.88). In patients who survived to discharge, referral to HF follow-up services differed between groups: patients with COPD-HF had reduced odds of cardiology follow-up (ORadj, 95% CI 0.79, 0.77 to 0.81), while cardiology referral odds for asthma-HF were similar to HF-alone. Overall, proportions of HF medication prescriptions at discharge were low for both COPD-HF and asthma-HF groups, particularly prescriptions for beta-blockers.
Conclusions
In this nationwide analysis, we showed that COPD and asthma significantly impact the clinical course in patients hospitalised for HF. COPD is associated with higher in-patient mortality and lower cardiology referral odds, while COPD and asthma are both associated with lower use of prognostic HF therapies on discharge. These data highlight therapeutic gaps and a need for better integration of cardiopulmonary services to improve healthcare provision for patients with HF and coexisting respiratory disease.
Keywords: heart failure, epidemiology, respiratory medicine (see Thoracic Medicine)
Strengths and limitations of this study.
This study evaluates the association between respiratory disease and in-hospital mortality and management outcomes in patients hospitalised with heart failure (HF) in a representative population from England and Wales.
HF diagnosis was based either on diagnostic tests or clinical investigations which limited misclassification bias.
HF with preserved ejection fraction was an exclusion diagnosis (ie, defined as HF in patients who did not have reduced ejection fraction) due to lack of information regarding specific diagnostic tests to confirm preserved ejection fraction status.
There was a large proportion of missing data regarding bronchodilators and inhaled corticosteroid prescriptions which prevented evaluation of their impact on outcomes.
Introduction
Chronic obstructive pulmonary disease (COPD) and asthma frequently coexist with heart failure (HF) and are independently associated with mortality and increased healthcare use.1 2 This has been explained by shared systemic inflammation, worsened by the presence of pulmonary disease as well as suboptimal HF management.3 Evidence suggests that patients with HF and comorbid COPD are less likely to receive guideline recommended treatment for their HF. For example, beta-blockers which are used in the management of HF with reduced ejection fraction (HFrEF) are often not prescribed to patients with COPD, due to fear of bronchoconstriction,4 reduced effectiveness of emergency beta-agonist medication, or difficulty in discriminating between COPD and asthma.5
Less data exist on the relationship between asthma and HF. Some studies have shown that asthma is associated with an increased occurrence of cardiovascular disease while others suggest this is limited to women or smokers6 and depends on age of asthma onset.7 This is further complicated by a component of chronic irreversible airflow obstruction in some people with long-standing asthma, associated with a reduced response to asthma therapy.8 This may, in turn, affect treatment choices in this group of patients and increase vulnerability to adverse events, versus either disease occurring alone. Meta-analyses and observational studies have suggested that the use of beta-agonists or inhaled corticosteroids in both COPD and asthma has been associated with HF-onset, HF-related hospitalisation and increase in cardiovascular events,9–11 which depend on disease severity and study setting, but nevertheless worsen prognosis.1 7
Our main aim was to compare in-hospital mortality and post-discharge HF management (referrals to HF services, discharge medication) among patients admitted to hospital with decompensated HF, with and without COPD or asthma in a sample of patients from the National Heart Failure Audit (NHFA) from England and Wales. We also investigated whether ejection fraction (EF) status affects outcomes in these patient groups.
Methods
We included patients older than 18 years of age admitted to hospital for HF between March 2012 and April 2018 whose data were submitted to the NHFA. We considered their first HF hospitalisation only (online supplemental methods, table 1).
bmjopen-2021-059122supp001.pdf (192.4KB, pdf)
Exposures
COPD was defined as having a history of COPD—chronic bronchitis and/or emphysema, confirmed by spirometry or beta agonist/steroid inhaler use.
Asthma was defined as having a history of childhood asthma and atopy or having an asthma diagnosis confirmed by a respiratory physician.
No diagnostic test results were provided for COPD or asthma (online supplemental table 2), and for the purposes of this work were based on being recorded as ‘yes’ (present) or ‘no’ (absent) in the audit data as defined previously.
EF status was defined as HFrEF and HF with preserved EF (HFpEF), determined through echocardiography, MRI, nuclear scan or angiogram. Those with an EF <40% were categorised as HFrEF. Due to a lack of information regarding specific diagnostic tests required to make a HFpEF diagnosis, we determined HFpEF as patients not categorised as HFrEF.12
Covariates were age, sex, New York Heart Association (NYHA) classification and place of care (cardiology ward vs other place of care (ie, general ward)) and comorbidities (atrial fibrillation (AF), ischaemic heart disease (IHD), diabetes, valve disease, hypertension (online supplemental table 2)).
Outcomes
Our primary outcome was in-hospital death during the index event (HF admission), defined as a dichotomised variable (died/alive at discharge), according to COPD or asthma status. Secondary analyses included post-discharge referral to HF services (cardiology, HF nurse, HF MDT (multidisciplinary team)) and prescriptions for HF medications at discharge in those with HFrEF.
Statistical analysis
Differences in baseline characteristics between patients with COPD-HF/asthma-HF and HF-alone are presented using percentages for categorial variables and medians and IQRs for continuous variables. We assessed differences between groups using χ2 and Kruskal-Wallis tests. We assessed differences in outcomes between patients with COPD-HF compared with HF-alone and between asthma-HF compared with HF-alone using multilevel logistic regression with a random effect for hospital, to calculate ORs and 95% CIs (online supplemental methods). In the main analysis, we adjusted for confounders with less than 20% missing data: age, sex, comorbidities, place of care and NYHA status. The model building process is presented in online supplemental table 2. Analyses of referrals were conducted similarly and excluded patients who died in-hospital. Associations between COPD or asthma and HF medication prescriptions at discharge (beta-blockers, ACE inhibitors (ACEis), angiotensin receptor blockers (ARBs) and mineralocorticoid receptor antagonists (MRAs)) excluded those with HFpEF.
Sensitivity analyses
Due to potential confounding, smoking and body mass index (BMI) were multiply imputed using a multilevel approach (online supplemental methods). We also repeated the main analysis in a cohort of patients including only a ‘confirmed HF diagnosis’ (ICD-10 HF diagnosis confirmed by imaging/BNP testing or adjudicated by a clinician in the absence of echocardiography).13
Analyses were performed with R V.4.0.3.
Results
Baseline characteristics are presented in table 1. In total, 217 329 patients were admitted to hospital in England–Wales due to decompensated HF between 2012 and 2018, with data on COPD/asthma status available (online supplemental figure 1). The median age was 81 years (IQR 72–87) and 53.7% were male. In-hospital death occurred in 12% of patients. COPD was diagnosed in 15% of patients and asthma in 6.6%. Most patients were characterised by either marked or severe breathlessness and half had a recorded HF management plan in place at discharge. Length of stay and deprivation ranking did not differ significantly between patients with COPD-HF, asthma-HF and HF-alone. Patients with COPD-HF were mostly male, were less often admitted to cardiology and were more frequently diagnosed with IHD compared with those with HF-alone; hypertension was slightly less common among patients with COPD-HF, whereas diabetes was more common. The proportion of patients with HFpEF was marginally higher in the COPD-HF group, compared with the HF-only group. Patients with asthma-HF were mostly female, with higher levels of diabetes and hypertension compared with HF-only. Conversely, AF was less common in the asthma-HF compared with the HF-alone group; there were also more patients with HFpEF.
Table 1.
Baseline characteristics according to COPD and asthma status in patients hospitalised for HF in England–Wales
| HF-alone (N=170 297) | COPD +HF (N=32 695) | Asthma +HF (N=14 400) | Overall (N=2 17 392) | |
| Age, median (IQR) | 81 (72, 88) | 79 (72, 85) | 79 (69, 86) | 81 (72, 87) |
| Missing | 67 (0.1%) | 22 (0.1%) | 10 (0.1%) | 199 (0.1%) |
| Male | 91 837 (53.9%) | 19 072 (58.3%) | 5936 (41.2%) | 116 845 (53.7%) |
| Missing | 74 (0.1%) | 44 (0.1%) | 21 (0.1%) | 239 (0.1%) |
| Place of admission | ||||
| Cardiology | 76 428 (44.9%) | 12 361 (37.8%) | 6147 (42.7%) | 94 936 (43.7%) |
| Other | 93 358 (54.8%) | 20 246 (61.9%) | 8218 (57.1%) | 21 822 (56.0%) |
| Missing | 511 (0.3%) | 88 (0.3%) | 35 (0.2%) | 634 (0.3%) |
| Died in-hospital | 20 316 (11.9%) | 4181 (12.8%) | 1337 (9.3%) | 25 834 (11.9%) |
| Device therapy | ||||
| None | 147 485 (86.6%) | 28 962 (88.6%) | 12 818 (89.0%) | 189 265 (87.1%) |
| CRT-D | 3047 (1.8%) | 496 (1.5%) | 189 (1.3%) | 3732 (1.7%) |
| CRT-P | 1681 (1%) | 296 (0.9%) | 142 (1%) | 2119 (1.0%) |
| ICD | 3001 (1.8%) | 511 (1.6%) | 211 (1.5%) | 3 723 (1.7%) |
| Missing | 15 083 (8.9%) | 2430 (7.4%) | 1040 (7.2%) | 18 553 (8.5%) |
| Comorbidities | ||||
| Valve disease | 38 213 (22.4%) | 7005 (21.4%) | 2906 (20.2%) | 48 124 (22.1%) |
| Missing | 3426 (2.0%) | 822 (2.5%) | 335 (2.3%) | 4583 (2.1%) |
| IHD | 65 992 (38.8%) | 14 198 (43.4%) | 5175 (35.9%) | 85 365 (39.3%) |
| Missing | 3667 (2.2%) | 811 (2.5%) | 335 (2.3%) | 4813 (2.2%) |
| Hypertension | 91 477 (53.7%) | 16 838 (51.5%) | 8208 (57%) | 116 523 (53.6%) |
| Missing | 1326 (0.8%) | 381 (1.2%) | 125 (0.9%) | 1832 (0.8%) |
| Diabetes | 50 194 (29.5%) | 10 348 (31.7%) | 4772 (33.1%) | 65 314 (30%) |
| Missing | 459 (0.3%) | 142 (0.4%) | 54 (0.4%) | 655 (0.3%) |
| AF | 72 235 (42.4%) | 13 728 (42%) | 5508 (38.2%) | 91 471 (42.1%) |
| Breathlessness (NYHA) | ||||
| No limitation of physical activity | 12 273 (7.2%) | 1254 (3.8%) | 768 (5.3%) | 14 295 (6.6%) |
| Slight limitation of ordinary physical activity | 24 541 (14.4%) | 3951 (12.1%) | 1993 (13.8%) | 30 485 (14.%) |
| Marked limitation of ordinary physical activity | 68 179 (40%) | 13 671 (41.8%) | 6011 (41.7%) | 87 861 (40.4%) |
| Symptoms at rest or minimal activity | 54 652 (32.1%) | 12 191 (37.3%) | 4809 (33.4%) | 71 652 (33%) |
| Missing | 10 652 (6.3%) | 1628 (5.0%) | 819 (5.7%) | 13 099 (6.0%) |
| Echocardiography performed | 137 955 (81%) | 26 165 (80%) | 11 342 (78.8%) | 175 462 (80.7%) |
| Ejection fraction status | ||||
| HFrEF | 92 619 (54.4%) | 16 408 (50.2%) | 7334 (50.9%) | 116 361 (53.5%) |
| HFpEF | 77 678 (45.6%) | 16 287 (49.8%) | 7066 (49.1%) | 101 031 (46.5%) |
| HF management plan | ||||
| Pre-discharge management plan in place | 11 760 (6.9%) | 2152 (6.6%) | 1002 (7.0%) | 14 914 (6.9%) |
| Management plan has been discussed with the patient | 10 572 (6.2%) | 1894 (5.8%) | 954 (6.6%) | 13 420 (6.2%) |
| Management plan has been communicated to the primary care team | 19 880 (11.7%) | 3963 (12.1%) | 1780 (12.4%) | 25 623 (11.8%) |
| All of the above | 83 507 (49%) | 15 496 (47.4%) | 7140 (49.6%) | 106 143 (48.8%) |
| No plan | 18 021 (10.6%) | 3937 (12.0%) | 1546 (10.7%) | 23 504 (10.8%) |
| Missing | 26 557 (15.6%) | 5253 (16.1%) | 1978 (13.7%) | 33 788 (15.5%) |
| Referral to HF MDT | 53 898 (31.6%) | 9719 (29.7%) | 4455 (30.9%) | 68 072 (31.3%) |
| Missing | 29 946 (17.6%) | 5722 (17.5%) | 2216 (15.4%) | 37 884 (17.4%) |
| Referral to cardiology follow-up | 70 925 (41.6%) | 11 875 (36.3%) | 6241 (43.3%) | 89 041 (41%) |
| Missing | 13 827 (8.1%) | 2882 (8.8%) | 984 (6.8%) | 17 693 (8.1%) |
| Referral to HF nurse follow-up | 76 170 (44.7%) | 13 728 (42.0%) | 6249 (43.4%) | 96 147 (44.2%) |
| Missing | 13 442 (7.9%) | 2658 (8.1%) | 952 (6.6%) | 17 052 (7.8%) |
| LOS | ||||
| Median (IQR) | 8 (3, 15) | 8 (4, 16) | 7 (3, 14) | 8 (4, 15) |
| IMD Wales (quartile) | N=8205 | N=1889 | N=776 | N=N=10 870 |
| 1st (most deprived) | 2126 (25.9%) | 371 (19.6%) | 188 (24.2%) | 2685 (24.7%) |
| 2nd | 2058 (25.1%) 396 | (21.0%) 190 | (24.5%) 83 | 2644 (24.3%) |
| 3rd | 1977 (24.1%) | 459 (24.3%) | 196 (25.3%) | 2632 (24.2%) |
| 4th (least deprived) | 1824 (22.2%) | 607 (32.1%) | 185 (23.8%) | 2616 (24.1%) |
| Missing* | – | – | – | – |
| IMD England (quartile) | N=1 59 540 | N=30 352 | N=13 433 | N=203 325 |
| 1st (most deprived) | 35 836 (22.5%) | 9338 (30.8%) | 3449 (25.7%) | 48 623 (23.9%) |
| 2nd | 38 347 (24.0%) | 7762 (25.6%) | 3403 (25.3%) | 49 512 (24.4%) |
| 3rd | 40 131 (25.2%) | 6848 (22.6%) | 3166 (23.6%) | 50 145 (24.7%) |
| 4th (least deprived) | 41 387 (25.9%) | 5615 (18.5%) | 3072 (22.9%) | 50 074 (24.6%) |
| Missing | 3839 (2.4%) | 789 (2.6%) | 343 (2.6%) | 4971 (2.4%) |
*Not shown due to small numbers.
AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; CRT-D, cardiac resynchronisation therapy defibrillator; CRT-P, cardiac resynchronisation therapy pacemaker; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter defibrillator; IHD, ischaemic heart disease; IMD, indices of multiple deprivation; LOS, length of stay; MDT, multidisciplinary team; NYHA, New York Heart Association.
In-hospital death
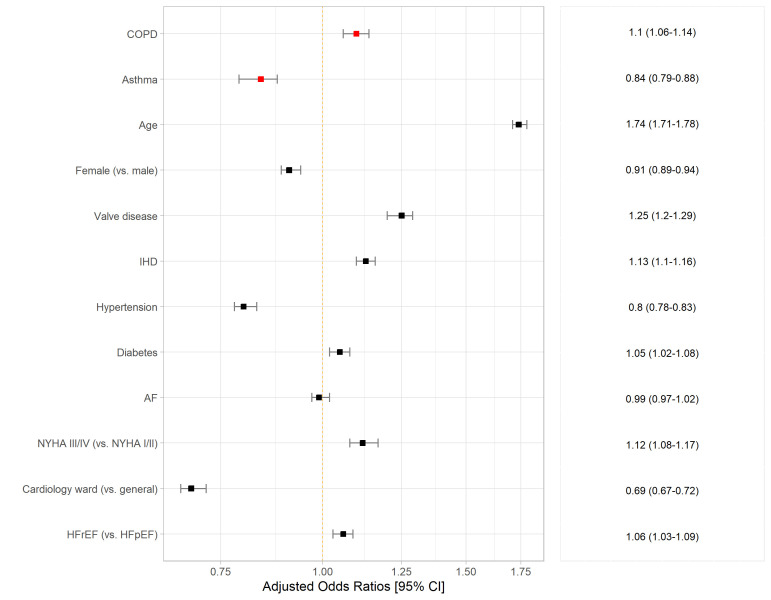
The association between COPD and in-hospital death is presented in figure 1, table 2 and online supplemental table 4. Overall, COPD was independently associated with increased odds of in-hospital death ((adjusted)ORadj, 95% CI: 1.10, 1.06 to 1.14). The relationship between COPD and in-hospital death differed according to EF: COPD was associated with an increase in mortality in patients with HFrEF (ORadj: 1.15, 1.09 to 1.21), but not in those with HFpEF (ORadj: 1.05, 0.99 to 1.10).
Figure 1.
Association between COPD, asthma and in-hospital death, adjusted for age, sex, valve disease; IHD, hypertension, diabetes, AF, NYHA, place of care and EF status; OR with 95% CIs. AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IHD, ischaemic heart disease; NYHA, New York Heart Association.
Table 2.
Association between COPD, asthma and outcomes in patients hospitalised for HF in England–Wales
| Fully adjusted* interaction model COPD×EF OR (95% CI) |
Fully adjusted† interaction model asthma×EF OR (95% CI) |
|||
| Outcome | COPD×HFrEF | COPD×HFpEF | Asthma×HFrEF | Asthma×HFpEF |
| In-hospital death (N=194 156‡) |
Interaction p value=0.01 | Interaction p value=0.842 | ||
| Fixed effects | 1.15 (1.09 to 1.21, p=0.294×10−10) | 1.05 (0.99 to 1.10, p=0.081) | – | – |
| Random effects (hospitals, n=216) | ||||
| Variance | 0.201 | – | ||
| LR test p value§ | p=0.22×10−16 | – | – | |
| Referral to cardiology follow-up (N=166 658‡) |
Interaction p value=0.288×10−7 | Interaction p value=0.0001 | ||
| Fixed effects | 0.85 (0.81, 0.88, p=0.2×10−16) | 0.73 (0.70, 0.76, p=0.2×10−16) | 1.08 (1.03 to 1.14, p=0.2×10−16) | 0.93 (0.88 to 0.98, p=0.003) |
| Random effects (hospitals, n=216) | ||||
| Variance | 0.512 | 0.512 | ||
| LR test p value§ | 0.22×10−16 | p=0.22×10−16 | ||
| Referral to HF MDT (N=149 098‡) |
Interaction p value=0.017 | Interaction p value=0.095 | ||
| Fixed effects | 0.97 (0.93, 1.02, p=0.263) | 0.90 (0.86, 0.94, p=0.265×10−5) | – | – |
| Random effects (hospitals, n=216) | ||||
| Variance | 2.139 | – | – | |
| LR test p value§ | 0.22×10−16 | – | – | |
| Referral to HF nurse (N=166 723‡) |
Interaction p value=0.249 | Interaction p value=0.450 | ||
*Adjusted for age, sex, diabetes, hypertension, ischaemic heart disease, atrial fibrillation, asthma, place of care and New York Heart Association status.
†Adjusted for age, sex, diabetes, hypertension, ischaemic heart disease, atrial fibrillation, COPD, place of care and New York Heart Association status.
‡Excludes patients with missing data on covariates included in model.
§Likelihood ratio test comparing fixed to random effects for hospital model fit, significant indicates random-effects model performed better than fixed-effects model.
COPD, chronic obstructive pulmonary disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LR, likelihood ratio; MDT, multidisciplinary team.
Conversely, asthma was associated with a decrease in the odds of in-hospital death compared with HF patients without asthma (ORadj, 95% CI: 0.85, 0.79 to 0.88). The odds of death did not vary by EF status for patients with asthma-HF (figure 1, table 2).
Sensitivity analyses where smoking status and BMI were imputed due to missing data (online supplemental table 5), and where patients with a confirmed HF diagnosis only were included (online supplemental table 6), showed similar results to the main analysis.
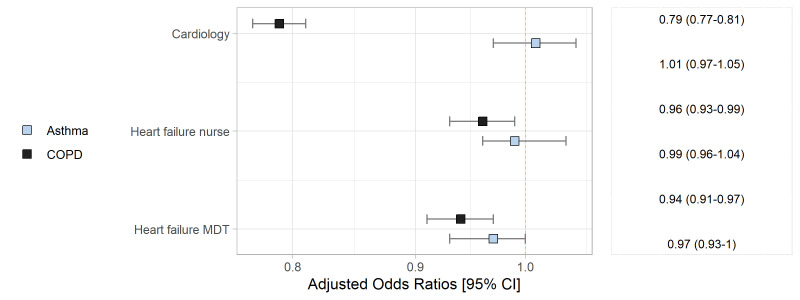
Referrals to HF services
In the fully adjusted models, COPD was associated with decreased likelihood of outpatient referral to a cardiologist (ORadj, 95% CI 0.79, 0.77 to 0.81) and to a HF-MDT (ORadj, 95% CI 0.94, 0.91 to 0.97). Patients with COPD-HFrEF were less likely to be referred to a cardiologist than those with HFrEF without COPD (ORadj: 0.85, 95% CI 0.81 to 0.88) while patients with COPD-HFpEF were significantly less likely to be referred, compared with HFpEF without COPD (ORadj, 95 CI% 0.73, 0.70 to 0.76). COPD was associated with a decreased likelihood of documented HF-MDT referral only for patients with HFpEF (ORadj, 95% CI, 0.90, 0.86 to 0.94).
Overall, referral odds did not differ in patients with asthma-HF compared with those with HF-alone. There was a significant increase in the odds of referral to a cardiologist for those with asthma-HFrEF (ORadj, 95% CI 1.08, 1.03 to 1.14) and a decreased likelihood of referral for patients with asthma-HFpEF (ORadj 95 CI, 0.93, 0.88 to 0.98), compared with HFrEF, HFpEF-alone, respectively. Referrals to HF nurse or HF MDT were not different between those with HF-alone or HF and asthma (figure 2).
Figure 2.
Association between chronic obstructive pulmonary disease (COPD), asthma and referrals to heart failure services, adjusted for age, sex, valve disease; ischaemic heart disease, hypertension, diabetes, atrial fibrillation, New York Heart Association, place of care and ejection fraction status. MDT, multidisciplinary team.
HF medication prescription at discharge
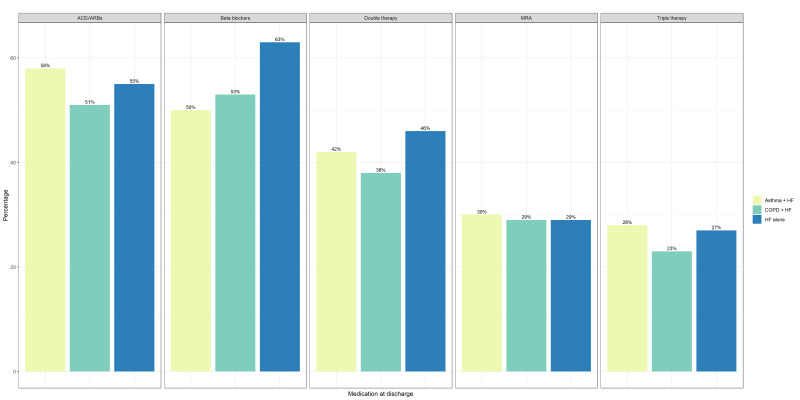
Patients with COPD-HF had lower prescription proportions of ACEIs/ARBs, beta-blockers and double (ACEi/ARB+beta-blocker) and triple therapy (ACEi/ARB+beta-blocker+MRA) compared with those with HF-only. ACEIs/ARBs, MRAs and triple therapy were prescribed more frequently in the asthma-HF group compared with the HF-alone group; however, beta-blockers or double therapy were less often prescribed for asthma-HF versus HF-alone (figure 3).
Figure 3.
Heart failure (HF) medication prescription rates at discharge, according to comorbid respiratory disease status. ACEi, ACE Angiotensin-converting enzyme; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; MRA, mineralocorticoid receptor antagonist.
In patients with HFrEF, COPD and asthma were associated with decreased likelihood of beta-blocker prescription at discharge (ORadj 0.66, 95% CI 0.59 to 0.67, ORadj: 0.57, 95% CI 0.54 to 0.60). COPD was associated with lower chance of ACEi/ARB prescription, but did not affect MRA prescriptions, while asthma was associated with increased odds of ACEi/ARB and MRA (table 3).
Table 3.
Association between COPD, asthma and HF medication prescription at discharge in patients with HFrEF
| Medication prescription at discharge | COPD unadjusted OR (95% CI) |
COPD fully adjusted* OR (95% CI) |
Asthma unadjusted OR (95% CI) |
Asthma fully adjusted† OR (95% CI) |
| Beta-blockers (N=86 449*,†) | ||||
| Fixed effects | 0.61 (0.58, 0.64, p=0.22×10−16) | 0.66 (0.64, 0.68, p=0.22×10−16) | 0.63 (0.59, 0.67, p=0.22×10−16) | 0.57 (0.54 0.60, p=0.22×10−16) |
| Random effects | ||||
| Variance | 0.553 | 0.578 | 0.549 | 0.578 |
| LR test p value | 0.22×10−16 | 0.22×10−16 | 0.22×10−16 | 0.22×10−16 |
| ACEis/ARBs (n=96 080*,†) | ||||
| Fixed effects | 0.87 (0.84, 0.90, p=0.139×10−13) | 0.91 (0.87 to 0.95, p=0.256×10−6) | 1.13 (1.07, 1.19, p=0.16×10−6) | 1.07 (1.01, 1.13, p=0.0143) |
| Random effects | ||||
| Variance | 0.149 | 0.130 | 0.148 | 0.130 |
| LR test p value | 0.22×10−16 | 0.22×10−16 | 0.22×10−16 | 0.22×10−16 |
| MRA (N=96 080*,†) | ||||
| Fixed effects | 0.97 (0.94, 1.01, p=0.114) | 1.02 (0.98, 1.06, p=0.268) | 1.08 (1.04, 1.13, p=0.00043) | 1.07 (1.02, 1.12, p=0.0084) |
| Random effects | ||||
| Variance | 0.232 | 0.195 | 0.226 | 0.195 |
| LR test p value | 0.22×10−16 | 0.22×10−16 | 0.22×10−16 | 0.22×10−16 |
Likelihood ratio test comparing fixed to random effects for hospital model fit, significant indicates random-effects model performed better than fixed-effects model.
*Adjusted for age, sex, diabetes, hypertension, ischaemic heart disease, atrial fibrillation, asthma, place of care and New York Heart Association status.
†Adjusted for age, sex, diabetes, hypertension, ischaemic heart disease, atrial fibrillation, COPD, place of care and New York Heart Association status
ACEi, ACE inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; HFrEF, heart failure with reduced ejection fraction; LR, likelihood ratio; MRA, mineralocorticoid receptor antagonist.
Discussion
This is the first study to provide a large assessment of contemporary HF practice, generalisable to the population of England–Wales, evaluating the effect of COPD and asthma on clinical and management outcomes. We found that patients with COPD-HF were more likely to die during their HF admission, compared with patients with HF-only; those with asthma-HF had a reduced probability of in-hospital death, compared with patients with HF-alone. Referrals to HF services also differed: COPD was associated with a 21% reduction in post-discharge cardiology referral while a diagnosis of asthma did not affect this outcome.
Airways diseases, particularly COPD, are associated with adverse events in patients with HF1–3 14–16; however, diagnostic misclassification is underestimated and studies on the independent effect of asthma are lacking. We report several findings which add to previous literature.
The finding that COPD is associated with in-hospital mortality confirms reports from previous European data which considered longer term follow-ups.17 18 A greater severity of cardiovascular disease among those with COPD-HF may have contributed to the increase in mortality, as indicated by the higher proportions of patients in NYHA classes III and IV, compared with those with HF-alone. Further explanations could include admission to non-cardiology wards for patients with COPD-HF, which has been linked to poorer outcomes in acute HF.19
A COPD diagnosis was associated with increased in-hospital death in those with HFrEF, but not in those with HFpEF, which is surprising, given that COPD is suggested to be more severe in the latter group.20 In contrast with our report, previous studies found that risk of death is increased in those with COPD-HFpEF compared with COPD-HFrEF21 22; however, these may be confounded by a lack of validity of EF status (inferred by ICD codes rather than echocardiography) or spirometry to confirm COPD status, consideration of long-term rather than short-term effects on mortality, or by including chronic rather than hospitalised HF. Our result therefore may be explained by poor uptake of disease-modifying treatments available for HFrEF in those with COPD,17 which has been previously reported and could be more pronounced in a cohort of patients newly admitted for HF.
After adjusting for age, sex and other baseline characteristics including comorbidities, and further adjustments for smoking status and BMI, differences between those with and without COPD, respectively asthma, did not materially change the association between the two lung diseases with in-hospital mortality. This suggests an independent contribution of COPD to increased mortality in patients hospitalised with HF, significant beyond the potential confounders considered in this analysis.
While previous reports suggest that asthma is associated with increased risk of developing cardiovascular disease,6 no prior study has reported on the association between asthma and death during acute HF hospitalisation. We found that, on average, asthma was independently associated with a 24% reduction in risk of death in patients with HF. The mechanisms underlying this epidemiological association are unclear. Several factors may explain our result. Asthma management is reliant on anti-inflammatory agents such as inhaled corticosteroids (ICS), which have been linked to cardioprotective effects23 24 including lower all-cause mortality and lower risk of myocardial infarction (MI, a precursor to HF). Potential long-term ICS use in our asthma-HF cohort could have diminished patients’ baseline mortality risk.
The nature of inflammation is different in COPD compared with asthma and influences response to medication. One hypothesis which may underlie the diverging findings on the effect of the two lung diseases on outcomes in patients with HF thus relates to differences in management and their subsequent differential cardiovascular risk. Bronchodilator medications, which are central to the symptomatic treatment of COPD, have been associated with increased cardiovascular risk.9 While combination treatments such as ICS/long-acting beta-agonists (LABA) may have a good cardiovascular safety profile in asthma, this differs in COPD.8 16 Randomised controlled trials have not demonstrated mortality benefits with ICS in individuals with COPD, although some observational studies suggest the opposite. The largest trial25 examining all-cause mortality in 16 000 patients with COPD and risk of cardiovascular disease showed that the treatments evaluated (LABA and/or ICS) were well tolerated by patients; however, the effect on patients with existing HF remains under debate.
Since both lung diseases were diagnosed prior to HF admission, it would be plausible to assume that any effects of long-term pulmonary medication could influence the chance of death in our cohort. Thus, the heightened risk of in-hospital mortality observed in the COPD-HF group, but not in asthma-HF, could be related to more frequent use of bronchodilators and a poorer safety profile of ICS in COPD compared with asthma. Alternatively, COPD-specific characteristics such as progressive lung function decline may have influenced in-hospital mortality in those admitted for HF.
However, due to large amounts of missing data on respiratory disease medication prescription in our cohort (online supplemental table 7), we could not verify these assumptions in our dataset. Future studies incorporating accurate information on bronchodilator use in patients with concomitant HF and respiratory disease should be conducted.
The association between COPD/asthma and referral to follow-up cardiology services has not been studied before in hospitalised patients with HF. Overall, patients with COPD-HF were less likely to be referred to a cardiology service after hospital discharge, compared with those who had HF-alone. This indicates that a COPD diagnosis may be an obstacle preventing access to HF specialist care. According to NICE, all patients with a HF diagnosis need to be seen by a HF specialist within 2 weeks of discharge, but data suggest that these timelines are not met.26 The compounded effect of a COPD diagnosis has the potential to further impair the long-term prognosis of these comorbid patients. Furthermore, more than 60% of patients with COPD and HF were admitted to a general ward rather than a specialised cardiology ward, which may also explain the low likelihood of cardiology referrals in this group.
Our study also indicated that EF status mediated the relationship with referrals, as individuals with COPD-HFpEF were less likely to have an appointment compared with their COPD-HFrEF counterparts. This is particularly worrying as HF, irrespective of EF, is best monitored and managed within specialist HF teams.
Asthma did not adversely influence referrals to HF services, but we identified an increased likelihood of referral to cardiology in asthma-HFpEF as compared with asthma-HFrEF. One possible explanation is greater uncertainty in clinical management of patients with HFpEF, leading to increased referral, although this needs to be assessed in future studies. Clarifying these clinical management pathways offers a potential to improve HF prognosis by ensuring access to care is timely and tailored to individual patients’ risk, pathology and health.
Patients with COPD-HFrEF were 34% less likely to receive a beta-blocker prescription at discharge, compared with patients with HFrEF alone, despite recent data supporting use of these agents in COPD.27 28 Similar to data on patients post-MI,29 it is worrying that COPD was also associated with decreased likelihood of guideline recommended ACEi/ARB prescription in those with HF, as there is no contraindication for those with pulmonary disease. Efforts need to be made to ensure appropriate therapeutic management of these patients.
Those with asthma-HFrEF had 43% less chance of being prescribed a beta-blocker compared with patients with HF-alone. Current guidelines recommend that asthma patients with chronic HFrEF should not receive disease-modifying beta-blocker treatment due to possible bronchoconstriction, despite evidence to suggest that cardioselective beta-blockade may be used with careful up-titration and monitoring,30 31 where benefits may outweigh risks in individual patients. Based on the low uptake across the whole spectrum of HF medications in patients with additional lung disease (figure 3), we expect these patients would have worse prognosis compared with their more adequately treated counterparts.
Considering these results, management needs to be optimised in patients with COPD or asthma and concurrent HF. The arrival of new treatments such as sodium-glucose co-transporter 2 inhibitors (SGLT2-i) have widened treatment choice in HFrEF, and there is now evidence supporting their use in individuals with COPD.32 Given beta-blockers are avoided in asthma, these new treatments should urgently be assessed in this population, as data are currently lacking.
Strengths and limitations
The main strength of this study is the large sample size and representativeness of a hospitalised population with HF from England and Wales. We did not, however, have information on duration and severity of asthma or COPD, nor lung function test results and thus we could not verify accuracy of these diagnoses, which are often subject to misclassification, especially in the elderly.33 Data on bronchodilator use was largely missing for our cohort (online supplemental table 7), limiting assessment of both diagnostic accuracy of the respiratory diseases, and association with outcomes evaluated in this study. We also could not differentiate between childhood asthma or late-onset asthma which may have different implications.34
HFpEF was determined as a HF diagnosis without systolic dysfunction, which has been used in previous NHFA reports. Nevertheless, there is no consensus gold standard HFpEF diagnosis12 and it remains difficult to validate. Further work in this area is needed, particularly in accurately distinguishing between HFpEF and COPD, which have similar clinical presentation.
There was a considerable proportion of missing data on bronchodilators/inhaled corticosteroids in the dataset which prevented assessment of whether the impact of COPD and asthma on outcomes is mediated, in part, by their treatment. Future studies incorporating accurate information on bronchodilator use in patients with concomitant HF and respiratory disease should be conducted.
Smoking status was also characterised by a large percentage of missing data; however, an analysis using multiple imputation indicated that even after adjusting for this confounder in the imputed dataset, the association between both COPD and asthma on in-hospital mortality remained unchanged (online supplemental table 5).
We only focused on decompensated HF and the picture may change when investigating long-term mortality, recurrent admissions or other aspects of treatment such as medication adherence.
While the referral likelihood estimates provide a first glimpse into the association between COPD/asthma and potential healthcare service provision for patients with HF in England–Wales, we did not have access to data on concrete healthcare utilisation among our cohort.
Due to lack of data, we could not establish whether cause of death varied among the groups and whether the increased mortality associated with COPD was underlined by higher rates of respiratory versus cardiac or other disease.
Conclusion
This analysis adds to the growing body of evidence that COPD and asthma affect outcomes in patients with acute HF. Our data suggest that while COPD is a main contributor to in-hospital mortality and is associated with decreased referral to cardiology services among patients with HF, asthma does not negatively impact these outcomes. Both lung diseases are, however, responsible for significant decreases in the prescription of HF treatments at discharge, particularly beta-blockers. These findings highlight a need for better integration of cardiopulmonary services with an aim to tailor healthcare provision for these patients.
Supplementary Material
Footnotes
Contributors: Conceptualisation and methodology: CG, JKQ, CK. Original draft: CG. Editing and final approval: CG, JKQ, RZ, CK. Data curation and formal data analysis: CG. Data acquisition: JKQ. Guarantor: CG
Funding: CG is funded by a NHLI PhD studentship. Grant number: N/A.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data that support the findings of this study have been provided by the Healthcare Quality Improvement Partnership from the National Heart Failure Audit Programme, but restrictions apply to the availability of these data and so are not publicly available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involved analysis of pre-existing, de-identified data, thus it was exempt from Institutional Review Board approval.
References
- 1.Lawson CA, Mamas MA, Jones PW, et al. Association of medication intensity and stages of airflow limitation with the risk of hospitalization or death in patients with heart failure and chronic obstructive pulmonary disease. JAMA Netw Open 2018;1:e185489. 10.1001/jamanetworkopen.2018.5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulea C, Zakeri R, Quint JK. Impact of chronic obstructive pulmonary disease on readmission after hospitalization for acute heart failure: a nationally representative US cohort study. Int J Cardiol 2019;290:113–8. 10.1016/j.ijcard.2019.04.087 [DOI] [PubMed] [Google Scholar]
- 3.Hawkins NM, Virani S, Ceconi C. Heart failure and chronic obstructive pulmonary disease: the challenges facing physicians and health services. Eur Heart J 2013;34:2795–807. 10.1093/eurheartj/eht192 [DOI] [PubMed] [Google Scholar]
- 4.Lipworth B, Wedzicha J, Devereux G, et al. Beta-Blockers in COPD: time for reappraisal. Eur Respir J 2016;48:880–8. 10.1183/13993003.01847-2015 [DOI] [PubMed] [Google Scholar]
- 5.Baker JG, Wilcox RG. β-Blockers, heart disease and COPD: current controversies and uncertainties. Thorax 2017;72:271–6. 10.1136/thoraxjnl-2016-208412 [DOI] [PubMed] [Google Scholar]
- 6.Pollevick ME, Xu KY, Mhango G, et al. The relationship between asthma and cardiovascular disease: an examination of the Framingham offspring study. Chest 2021;159:1338-1345. 10.1016/j.chest.2020.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iribarren C, Tolstykh IV, Miller MK, et al. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. Am J Epidemiol 2012;176:1014–24. 10.1093/aje/kws181 [DOI] [PubMed] [Google Scholar]
- 8.Buist AS. Similarities and differences between asthma and chronic obstructive pulmonary disease: treatment and early outcomes. Eur Respir J Suppl 2003;39:30S–5. 10.1183/09031936.03.00404903 [DOI] [PubMed] [Google Scholar]
- 9.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest 2004;125:2309–21. 10.1378/chest.125.6.2309 [DOI] [PubMed] [Google Scholar]
- 10.Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med 2013;173:1175–85. 10.1001/jamainternmed.2013.1016 [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Loke YK, Enright PL, et al. Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trials. BMJ 2011;342:d3215. 10.1136/bmj.d3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–317. 10.1093/eurheartj/ehz641 [DOI] [PubMed] [Google Scholar]
- 13.The heart failure dataset for the National Audit of Heart failure. Available: https://www.nicor.org.uk/national-cardiac-audit-programme/datasets/ [Accessed 26 April 2021].
- 14.Hawkins NM, Petrie MC, Jhund PS, et al. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail 2009;11:130–9. 10.1093/eurjhf/hfn013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lainscak M, Anker SD. Heart failure, chronic obstructive pulmonary disease, and asthma: numbers, facts, and challenges. ESC Heart Fail 2015;2:103–7. 10.1002/ehf2.12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabe KF, Hurst JR, Suissa S. And COPD: dangerous liaisons? Eur Respir Rev 2018;27. 10.1183/16000617.0057-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canepa M, Straburzynska-Migaj E, Drozdz J, et al. Characteristics, treatments and 1-year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long-Term Registry. Eur J Heart Fail 2018;20:100–10. 10.1002/ejhf.964 [DOI] [PubMed] [Google Scholar]
- 18.Staszewsky L, Cortesi L, Tettamanti M, et al. Outcomes in patients hospitalized for heart failure and chronic obstructive pulmonary disease: differences in clinical profile and treatment between 2002 and 2009. Eur J Heart Fail 2016;18:840–8. 10.1002/ejhf.519 [DOI] [PubMed] [Google Scholar]
- 19.Gheorghiade M, Sopko G, De Luca L, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation 2006;114:1202–13. 10.1161/CIRCULATIONAHA.106.623199 [DOI] [PubMed] [Google Scholar]
- 20.Iversen KK, Kjaergaard J, Akkan D, et al. Chronic obstructive pulmonary disease in patients admitted with heart failure. J Intern Med 2008;264:361–9. 10.1111/j.1365-2796.2008.01975.x [DOI] [PubMed] [Google Scholar]
- 21.Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998–1005. 10.1016/j.jacc.2011.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Museedi AS, Alshami A, Douedi S, et al. Predictability of inpatient mortality of different comorbidities in both types of acute decompensated heart failure: analysis of national inpatient sample. Cardiol Res 2021;12:29–36. 10.14740/cr1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camargo CA, Barr RG, Chen R, et al. Prospective study of inhaled corticosteroid use, cardiovascular mortality, and all-cause mortality in asthmatic women. Chest 2008;134:546–51. 10.1378/chest.07-3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suissa S, Assimes T, Brassard P, et al. Inhaled corticosteroid use in asthma and the prevention of myocardial infarction. Am J Med 2003;115:377–81. 10.1016/S0002-9343(03)00393-0 [DOI] [PubMed] [Google Scholar]
- 25.Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet 2016;387:1817–26. 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- 26.Hayhoe B, Kim D, Aylin PP, et al. Adherence to guidelines in management of symptoms suggestive of heart failure in primary care. Heart 2019;105:678–85. 10.1136/heartjnl-2018-313971 [DOI] [PubMed] [Google Scholar]
- 27.Gulea C, Zakeri R, Quint JK. Effect of beta-blocker therapy on clinical outcomes, safety, health-related quality of life and functional capacity in patients with chronic obstructive pulmonary disease (COPD): a protocol for a systematic literature review and meta-analysis with multiple treatment comparison. BMJ Open 2018;8:e024736. 10.1136/bmjopen-2018-024736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salpeter SR, Ormiston TM, Salpeter EE, et al. Cardioselective beta-blockers for chronic obstructive pulmonary disease: a meta-analysis. Respir Med 2003;97:1094–101. 10.1016/S0954-6111(03)00168-9 [DOI] [PubMed] [Google Scholar]
- 29.Rothnie KJ, Smeeth L, Herrett E, et al. Closing the mortality gap after a myocardial infarction in people with and without chronic obstructive pulmonary disease. Heart 2015;101:1103–10. 10.1136/heartjnl-2014-307251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw SM, Williams SG. Should beta-blockade continue to be withheld from patients with chronic heart failure and asthma? Eur Heart J 2009;30:1287–87. 10.1093/eurheartj/ehp146 [DOI] [PubMed] [Google Scholar]
- 31.Gulea C, Zakeri R, Alderman V, et al. Beta-Blocker therapy in patients with COPD: a systematic literature review and meta-analysis with multiple treatment comparison. Respir Res 2021;22:64. 10.1186/s12931-021-01661-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canepa M, Ameri P, Lainscak M. Chronic obstructive pulmonary disease and comorbidities in heart failure: the next frontier of sodium-glucose co-transporter 2 inhibitors? Eur J Heart Fail 2021;23:644–7. 10.1002/ejhf.2109 [DOI] [PubMed] [Google Scholar]
- 33.Bloom CI, Nissen F, Douglas IJ, et al. Exacerbation risk and characterisation of the UK's asthma population from infants to old age. Thorax 2018;73:313–20. 10.1136/thoraxjnl-2017-210650 [DOI] [PubMed] [Google Scholar]
- 34.Ingebrigtsen TS, Marott JL, Vestbo J, et al. Coronary heart disease and heart failure in asthma, COPD and asthma-COPD overlap. BMJ Open Respir Res 2020;7:e000470. 10.1136/bmjresp-2019-000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059122supp001.pdf (192.4KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The data that support the findings of this study have been provided by the Healthcare Quality Improvement Partnership from the National Heart Failure Audit Programme, but restrictions apply to the availability of these data and so are not publicly available.