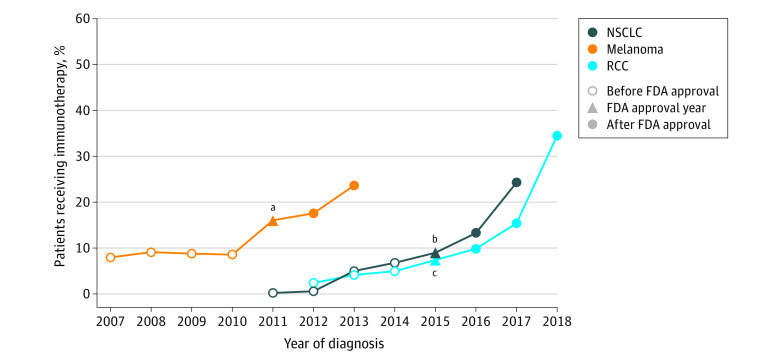
Figure 1. Receipt of Immunotherapy for Stage IV Cancer Over Time.
Among all patients who received immunotherapy within a diagnosis year. The Food and Drug Administration (FDA) approval year pertains to the year the first immune checkpoint inhibitor therapy for the respective cancer type was approved (eMethods in the Supplement). NSCLC indicates non–small cell lung cancer; RCC, renal cell carcinoma.
aFor melanoma, the FDA approval year was included in the post–FDA approval era. The pre–FDA approval era was 2007 to 2010, and the early post–FDA approval era was 2011 to 2013.
bFor NSCLC, the FDA approval year was included in the post–FDA approval era. The pre–FDA approval era was 2011 to 2014, and the early post–FDA approval era was 2015 to 2017.
cFor RCC, the FDA approval year was included in the pre–FDA approval era. The pre–FDA approval era was 2012 to 2015, and the early post–FDA approval era was 2016 to 2018.