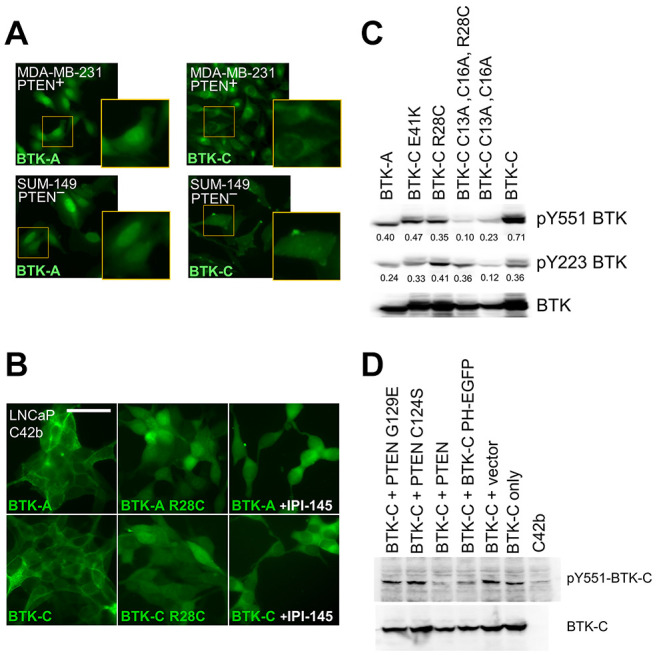
Figure 4. BTK isoform localization and activation is responsive to PI3K pathway signaling in solid tumor cells. (A) BTK isoform localization in MDA-MB-231 (PTEN+) and SUM149 (PTEN–) cells. (B) Pharmacological inhibition of PI3K decreases association of BTK-A and BTK-C with the plasma membrane in LNCaP C4-2b (PTEN–) cells. Scale bar: 100 μm. (C) Activating tyrosine phosphorylation of the BTK isoforms in transiently transfected HEK293 cells. Y551 phosphorylation is dependent on other kinases, Y223 autophosphorylation results from BTK activation. Values represent fold tyrosine-phosphorylated signal normalized to total transfected BTK control (anti-Flag) determined by densitometry. (D) PIP3 dependence of BTK-C activation in transfected LNCaP C4-2b (PTEN–) cells. PTEN activity by expression of PTEN dominant negative mutants increases BTK-C activation. Additionally, a BTK-C non-kinase domain construct (PH-EGFP) capable of dimerizing with full length BTK reduces activating phosphorylation. In each case, 24 hours after transfection cell lysates were collected for immunoblotting.