Abstract
Background
Despite the fact that the rate of type 1 diabetes (T1D) is increasing worldwide, there exists a dearth of information on the disease in most sub-Saharan African countries. The goal of this study was to determine the enrolment trend of T1D using data compiled over 28 y from a teaching hospital in Kumasi, Ghana.
Methods
Information collected included sex, age at diagnosis and date of T1D diagnosis. We identified trends from 1992 to 2018, divided into 3 y intervals.
Results
From 1992 to 2018, 1717 individuals with T1D were enrolled in the diabetes clinic at the Komfo Anokye Teaching Hospital. The male:female ratio was 1:1.2. The number of individuals diagnosed with T1D decreased among the 10–19 y age group during the 1992–1994 period, followed by a progressive increase within the same age group during the subsequent period (from 35.4% in 1995–1997 to 63.2% in 2016–2018). There was a decline in the proportion of children 0–9 y of age diagnosed during the study period (from 5.1% in 1992–1994 to 3.6% in 2016–2018).
Conclusions
In our study population, a decreasing trend of T1D enrolments was observed in general while among adolescents an increasing trend was observed.
Keywords: diabetes mellitus, enrolments, Ghana, Kumasi, trends, type 1
Introduction
Type 1 diabetes (T1D) is considered a public health threat due to the increasing number of patients, the early age of disease onset and the severity of complications associated with it.1,2 Globally, the incidence of T1D has increased by 2–5%.3 In the EURODIAB ACE Study (a collaboration of European childhood diabetes registers), an annual increase of 2.8% in T1D was recorded.2 Other epidemiological studies, including the Diabetes Mondiale study (DiaMond) and the Search for Diabetes in Youth (SEARCH) studies, reported similar findings.3,4 Despite increased efforts to understand the epidemiology of the disease, most sub-Saharan African countries lag behind in the areas of disease surveillance and diagnosis.5–8
The age of T1D diagnosis varies, with a high prevalence among teenagers and a relatively low prevalence among older age groups.9–11 In Europe, the USA and Israel for instance, the incidence of the disease is particularly high between the ages of 10 and 15 y.1,12,13 In Africa, Oceania and Japan, the disease has been reported to occur at later ages.14–17 Data on T1D epidemiology in Ghana are scanty and incomplete, despite the need for locally relevant data to guide policy and strategy. In this study we investigated the trend in enrolment of T1D over 28 y in a teaching hospital in Kumasi, Ghana.
Materials and methods
This retrospective study was approved by the Committee of Human Research and Ethics of the Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. The setting was the diabetes clinic of the Komfo Anokye Teaching Hospital, which serves as the main referral clinic for diabetes in Kumasi. Additionally, the hospital receives referrals from the middle and northern parts of Ghana. Records of patients with T1D who enrolled in the diabetes clinic from 1992 to 2018 were included. Subjects were diagnosed according to the American Diabetes Association criteria.18 Most of the patients were diagnosed using clinical indices such as age at diagnosis, physical appearance (i.e. weight and body mass index), presence of ketosis and the use of daily insulin injections. In cases with ambiguity, autoantibody screening together with C-peptide and insulin levels were determined to confirm or support the diagnosis. Patients with other types of diabetes (i.e. type 2 diabetes, monogenic diabetes, drug-induced diabetes and gestational diabetes) were excluded from the study.
Data management and statistical analysis
Using a structured, standardized data abstraction form, we extracted data including sex, age and date of diagnosis from the patients’ medical charts and the database at the diabetes clinic. We analysed data from 1992 to 2018, divided into 3 y intervals: 1992–1994, 1995–1997, 1998–2000, 2001–2003, 2004–2006, 2007–2009, 2010–2012, 2013-2015 and 2016-2018. We categorized the age at diagnosis into three groups: 0–9, 10–19 and ≥20 y. Data were analysed using the Statistical Package for the Social Sciences software version 23.0 (IBM, Armonk, NY, USA). Descriptive data were summarized using means, medians and ranges.
Results
A total of 1717 T1D patients qualified for inclusion in the study. Overall, 941 females (54.9%) and 776 (45.2%) males with T1D were enrolled between 1992 and 2018. Table 1 shows the distribution of age at diagnosis and sex. There was a peak in the diagnosis of T1D from 2007 to 2009. After an initial decrease in the proportion of individuals diagnosed within the 10–19 y age group (i.e. from 1992 to 1994 and 1994 to 1997), there was an increase during the subsequent periods through 2018. For those ≥20 y of age, there was a general decline in numbers over the period under study. The proportion of patients diagnosed within the 0–9 y age group remained fairly constant over the study period. The youngest age at diagnosis was 9 mo and the oldest was 40 y. The mean age at diagnosis was 19.2±3.97 and the median was 19 y (range 17–22.0). The enrolment trends are presented in Figure 1.
Table 1.
Distribution of T1D diagnosis from 1992 to 2018 by age at diagnosis and sex
| Age at diagnosis | All, n (%) | Sex, n (%) | |
|---|---|---|---|
| Male | Female | ||
| Children, 0–9 y | 61 (3.6) | 34 (55.7) | 27 (44.3) |
| Adolescents, 10–19 y | 837 (48.7) | 368 (44.0) | 469 (56.0) |
| Adults, ≥20 y | 819 (47.7) | 374 (45.7) | 445 (54.3) |
| Total, N | 1717 | 776 | 941 |
Figure 1.
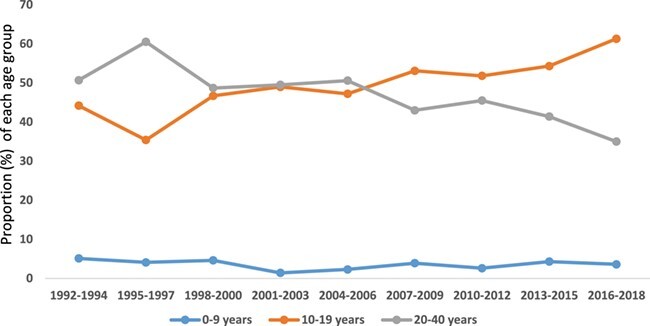
Trend of age at type 1 diabetes diagnosis in Ghana, 1992–2018.
As shown in Table 3, the number of males and females diagnosed with T1D peaked during the 1992–1994 period. From 1992 to 1994 there was an increase in the overall mean age at diagnosis for T1D. The mean age at diagnosis remained relatively stable, with a slight variation between 1995 and 2012 until a declining trend emerged from 2013 to 2018. The mean age among females was 19.08±3.76 y (median 19.00), while the mean age in males was 19.35±4.18 y (median 19.00). A detailed analysis of age trends at T1D diagnosis during each period is shown in Table 2.
Table 3.
Temporal trend of age at T1D diagnosis by sex
| Year of diagnosis | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Age at diagnosis (y), n (%) | Age at diagnosis (y), n (%) | |||||||
| 0–9 | 10–19 | 20–40 | Total | 0–9 | 10–19 | 20–40 | Total | |
| 1992–1994 | 9 (8.7) | 54 (51.9) | 41 (39.4) | 104 | 2 (1.8) | 41 (36.9) | 68 (61.3) | 111 |
| 1995–1997 | 3 (3.5) | 33 (38.4) | 50 (58.1) | 86 | 5 (4.6) | 36 (33.0) | 68 (62.4) | 109 |
| 1998–2000 | 4 (4.7) | 44 (51.2) | 38 (44.2) | 86 | 5 (4.6) | 47 (43.1) | 57 (52.3) | 109 |
| 2001–2003 | 1 (1.0) | 46 (46.5) | 52 (52.5) | 99 | 2 (1.8) | 57 (51.4) | 52 (46.8) | 111 |
| 2004–2006 | 2 (2.2) | 38 (42.2) | 50 (55.6) | 90 | 2 (2.3) | 45 (52.3) | 39 (45.3) | 86 |
| 2007–2009 | 4 (4.1) | 45 (45.9) | 49 (50.0) | 98 | 6 (3.8) | 92 (57.5) | 62 (38.8) | 160 |
| 2010–2012 | 4 (4.5) | 48 (53.9) | 37 (41.6) | 89 | 1 (1.0) | 51 (50.0) | 50 (49.0) | 102 |
| 2013–2015 | 3 (4.5) | 35 (52.2) | 29 (43.3) | 67 | 3 (4.1) | 41 (56.2) | 29 (39.7) | 73 |
| 2016–2018 | 4 (7.0) | 25 (43.9) | 28 (49.1) | 57 | 1 (1.2) | 59 (73.8) | 20 (25.0) | 80 |
Table 2.
Temporal trend of age at T1D diagnosis in Ghana
| Year of diagnosis | Age at diagnosis (y), n (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0–9 | 10–19 | 20–40 | Total, n | Mean (SD) | Minimum | Maximum | |
| 1992–1994 | 11 (5.1) | 95 (44.2) | 109 (50.7) | 215 | 19.29 (6.33) | 1 | 36 |
| 1995–1997 | 8 (4.1) | 69 (35.4) | 118 (60.5) | 195 | 20.40 (6.30) | 2 | 36 |
| 1998–2000 | 9 (4.6) | 91 (46.7) | 95 (48.7) | 195 | 19.43 (6.36) | 3 | 37 |
| 2001–2003 | 3 (1.4) | 103 (49.0) | 104 (49.5) | 210 | 18.92 (4.29) | 2 | 36 |
| 2004–2006 | 4 (2.3) | 83 (47.2) | 89 (50.6) | 176 | 19.13 (4.85) | 2 | 34 |
| 2007–2009 | 10 (3.9) | 137 (53.1) | 111 (43.0) | 258 | 19.31 (6.26) | 2 | 40 |
| 2010–2012 | 5 (2.6) | 99 (51.8) | 87 (45.5) | 191 | 19.72 (6.56) | 2 | 38 |
| 2013–2015 | 6 (4.3) | 76 (54.3) | 58 (41.4) | 140 | 17.97 (4.37) | 3 | 31 |
| 2016–2018 | 5 (3.6) | 84 (61.3) | 48 (35.0) | 137 | 17.72 (5.17) | 2 | 36 |
| Total, n (%) | 61 (3.6) | 837 (48.7) | 819 (47.7) | 1717 | |||
Discussion
Globally, the diagnosis of T1D has steadily increased.19 This is despite the incidence plateauing in countries such as Sweden, Finland and Ireland.20–23 Both the DiaMond and the EURODIAB ACE studies showed varying incidence rates among different age categories.2,3 The latest report from the EURODIAB ACE group, which analysed data from 1989 to 2013, showed a trend towards a high incidence of T1D during early childhood.1
Our study, despite showing a general decline in the overall incidence of T1D, also showed an increase in the 10–19-y age group. This finding corresponds to previous studies showing a high incidence of T1D, especially among the adolescent age group.2 The general decline in the incidence of the disease in our cohort, despite evidence to the contrary elsewhere, may have resulted from the study being a single-centre study. It may also reflect the iceberg phenomenon, with an underestimation of T1D diagnosis due to non-reporting, misclassification, misdiagnosis or death before diagnosis. To remedy this situation, intensive education of both healthcare professionals and the general public on the early signs of the disease needs to be employed. T1D diagnosis among the cohort was mostly done on clinical grounds. This may have contributed to the low numbers of T1D cases recorded for the period under study. Additionally, the reliance on clinical features vs definite biochemical confirmation may have led to some misclassification and an underestimation of the true burden of the disease. A lot more needs to be done to integrate definite diagnostic measures of T1D using immunological and other modalities in Kumasi to understand the true burden of the disease.
The reduction in T1D incidence as seen in our study can also be attributed to deterioration in environmental hygienic practices and the consequent high rates of various helminth infections among children and adolescents in Kumasi.24,25 Most helminths have been shown to impact positively in modifying the immune response in favour of decreasing T1D.24,25 Another reason for the decreasing trend may be the increase in human resource capacity at peripheral health facilities. Therefore T1D cases may not necessarily be referred to the Komfo Anokye Teaching Hospital as a central point.
As shown in this study, there was a trend towards an increasing mean age at diagnosis of T1D through 2013, when the mean age subsequently declined. The age at diagnosis of T1D varies worldwide, tending to be low in the USA and Europe and relatively high in Africa and Asia.14,26,27 The decline in the mean age at diagnosis in our study can be attributed to the increase in incidence in the younger age groups. The trend of increasing incidence of T1D during the adolescent years found in our study is similar to recent reports from the EURODIAB ACE study from the Netherlands that showed a high incidence of diagnosis within the 10–14 y age group.28 Harjutsalo et al.31 recorded an increase in the incidence of T1D in the 10–14 y age group (58.1 to 68.6 per 100 000 person-years) but a decline in the incidence of T1D in early childhood (i.e. the 0–4 y age group [57.3 to 52.8 per 100 000 person-years]) and the 5–9 y age group (80.6 to 73.0 per 100 000 person-years) recorded during the same period.
As well as environmental factors, epigenetic changes including modification by the gut microbiome have been studied and shown to be a contributing factor to the development of and an influence on the age of T1D diagnosis.30,31 Gradual social and environmental development within Ghana and a big city like Kumasi is usually associated with increased environmental sanitation, dietary changes and the use of antibiotics, as well as chemical pollution from industrialization. All these factors impact the diversity of the gut microbiome in infancy and childhood.32 Such microbiome differences in individuals with the same genotype could influence disease manifestation and time of onset, as well as explain how rapid changes in modern lifestyles could directly affect T1D incidence. Epigenetic modifications may also play a role in the development of epidemiological variations which partly determines the many environmental factors implicated in T1D, including dietary components, antibiotics and environmental pollutants.33
Limitations
Due to the retrospective nature of this study, it is impossible to draw any causal links to explain the trends identified. However, the trends described using historical data provide a good basis for future prospective research to explain the current trends identified. Additionally, the absence of data for several clinical indicators limited the depth of the analysis. Also, since the data analysed is from a single centre, the diabetes clinic of the Komfo Anokye Teaching Hospital, it cannot easily be generalized to the entire Ghanaian population. However, the contributing role played by the centre since its inception, serving as the central referral point for much of middle and northern Ghana, still makes the findings useful.
Conclusions
Our findings point to an increased incidence of T1D diagnosis among teenagers while the incidence is decreasing among other age groups. Since the same trend has been reported by other studies, it is an intriguing finding that merits further study. The combined effect of genetic predisposition, as well as environmental influences, makes it important to understand the role of changing diet, epidemiological shifts in disease patterns and improved diagnosis in the changes seen in the incidence of T1D across various age groups in the Ghanaian context.
Declaration of interests
None.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Authors’ contributions:
OS-K, MA-B, JA and NAB conceived the study. OS-K, MA-B, JA, NAB and EYT designed the study protocol. OS-K, MA, MA-B and JA supervised the data extraction. MA-B, EYT, JA, OS-K, MA, WM and NAB wrote the plan for the data analysis. MA-B carried out the data analysis. All authors interpreted the results of the data analysis. EYT, MA-B, OS-K, MM, MA and JA drafted and critically revised the manuscript for intellectual content. All authors approved the final manuscript. OS-K, MA-B and JA are guarantors of the paper.
Acknowledgements:
None.
Funding:
None.
Competing interests:
None declared.
Ethical approval:
This study was approved by the Committee on Human Research Publication and Ethics of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology and the Komfo Anokye Teaching Hospital, Kumasi, Ghana.
References
- 1. Maahs DM, West NA, Lawrence JM, et al. . Chapter 1: epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. EURODIAB Study Group . Variation and trends in incidence of childhood diabetes in Europe. Lancet. 2000;355(9207):873–6. [PubMed] [Google Scholar]
- 3. Karvonen M, Viik-Kajander M, Moltchanova E, et al. . Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23(10):1516–26. [DOI] [PubMed] [Google Scholar]
- 4. Dabelea D, Bell RA, D’AgostinoRB Jr, et al. . Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716–24. [DOI] [PubMed] [Google Scholar]
- 5. SEARCH Study Group . SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 2004;25(5):458–71. [DOI] [PubMed] [Google Scholar]
- 6. Mayer-Davis EJ, Bell RA, Dabelea D, et al. . The many faces of diabetes in American youth: type 1 and type 2 diabetes in five race and ethnic populations: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(Suppl 2):S99–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peer N, Kengne AP, Motala AA, et al. . Diabetes in the Africa region: 2013 update for the IDF Diabetes Atlas. Diabetes Res Clin Pract. 2014;103(2):197–205. [DOI] [PubMed] [Google Scholar]
- 8. Bell RA, Mayer-Davis EJ, Beyer JW, et al. . Diabetes in non-Hispanic white youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(Suppl 2):S102–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steyn NP, Mchiza ZJR, Kengne AP. Future challenges for pediatric diabetes management in developing countries: lessons from Africa. Exp Rev Endocrinol Metab. 2015;10(1):75–86. [DOI] [PubMed] [Google Scholar]
- 10. Patterson C, Guariguata L, Dahlquist G, et al. . Diabetes in the young – a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract. 2014;103(2):161–75. [DOI] [PubMed] [Google Scholar]
- 11. Thunander M, Petersson C, Jonzon K, et al. . Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract. 2008;82(2):247–55. [DOI] [PubMed] [Google Scholar]
- 12. Patterson CC, Dahlquist GG, Gyurus E, et al. . Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–33. [DOI] [PubMed] [Google Scholar]
- 13. Liese AD, D’Agostino RB Jr, Hamman RF, et al. . The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics 2006;118(4):1510–18. [DOI] [PubMed] [Google Scholar]
- 14. Lipman TH, Levitt Katz LE, Ratcliffe SJ, et al. . Increasing incidence of type 1 diabetes in youth: twenty years of the Philadelphia Pediatric Diabetes Registry. Diabetes Care. 2013;36(6):1597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haynes A, Bulsara MK, Bower C, et al. . Cyclical variation in the incidence of childhood type 1 diabetes in Western Australia (1985–2010). Diabetes Care. 2012;35(11):2300–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Onda Y, Sugihara S, Ogata T, et al. . Incidence and prevalence of childhood-onset type 1 diabetes in Japan: the T1D study. Diabet Med 2017;34(7):909–15. [DOI] [PubMed] [Google Scholar]
- 17. Padoa CJ. The epidemiology and pathogenesis of type 1 diabetes mellitus in Africa. J Endocrinol Metab Diabetes S Afr. 2011;16(3):130–6. [Google Scholar]
- 18. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karvonen M, Tuomilehto J, Libman I, et al. . A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1993;36(10):883–92. [DOI] [PubMed] [Google Scholar]
- 20. Harjutsalo V, Sund R, Knip M, et al. . Incidence of type 1 diabetes in Finland. JAMA. 2013;310(4):427–8. [DOI] [PubMed] [Google Scholar]
- 21. Berhan Y, Waernbaum I, Lind T, et al. . Thirty years of prospective nationwide incidence of childhood type 1 diabetes: the accelerating increase by time tends to level off in Sweden. Diabetes. 2011;60(2):577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roche EF, McKenna AM, Ryder KJ, et al. . Is the incidence of type 1 diabetes in children and adolescents stabilising? The first 6 years of a national register. Eur J Pediatr. 2016;175(12):1913–19. [DOI] [PubMed] [Google Scholar]
- 23. Skrivarhaug T, Stene LC, Drivvoll AK, et al. . Incidence of type 1 diabetes in Norway among children aged 0–14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian Childhood Diabetes Registry. Diabetologia. 2014;57(1):57–62. [DOI] [PubMed] [Google Scholar]
- 24. Amoah ID, Abubakari A, Stenström TA, Abaidoo RC, Seidu R. Contribution of wastewater irrigation to soil-transmitted helminths infection among vegetable farmers in Kumasi, Ghana. PLoS Negl Trop Dis. 2016;10(12):e0005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walana W, Aidoo ENK, Tay SCK. Prevalence of hookworm infection: a retrospective study in Kumasi. Asia Pac J Trop Biomed. 2014;4(Suppl 1):S158–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zaccone P, Hall SW. Helminth infection and type 1 diabetes. Rev Diabet Stud. 2012;9(4):272–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lachmandas E, Thiem K, Heuvel C, et al. . Patients with type 1 diabetes mellitus have impaired IL-1β production in response to Mycobacterium tuberculosis. Eur J Clin Microbiol Infect Dis. 2018;37(2):371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vehik K, Hamman RF, Lezotte D, et al. . Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30(3):503–9. [DOI] [PubMed] [Google Scholar]
- 29. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. . Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fazeli Farsani S, Souverein PC, Vorst MM, et al. . Increasing trends in the incidence and prevalence rates of type 1 diabetes among children and adolescents in the Netherlands. Pediatr Diabetes. 2016;17(1):44–52. [DOI] [PubMed] [Google Scholar]
- 31. Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371(9626):1777–82. [DOI] [PubMed] [Google Scholar]
- 32. Phillips JE, Couper JJ, Penno MA, et al. . Type 1 diabetes: a disease of developmental origins. Pediatr Diabetes. 2017;18(6):417–21. [DOI] [PubMed] [Google Scholar]
- 33. Awunyo-Akaba Y, Awunyo-Akaba J, Gyapong M, Senah K, Konradsen F, Rheinlander T. Sanitation investments in Ghana; an ethnographic investigation of the role of land tenure. BMC Public Health. 2016;16:594. [DOI] [PMC free article] [PubMed] [Google Scholar]


