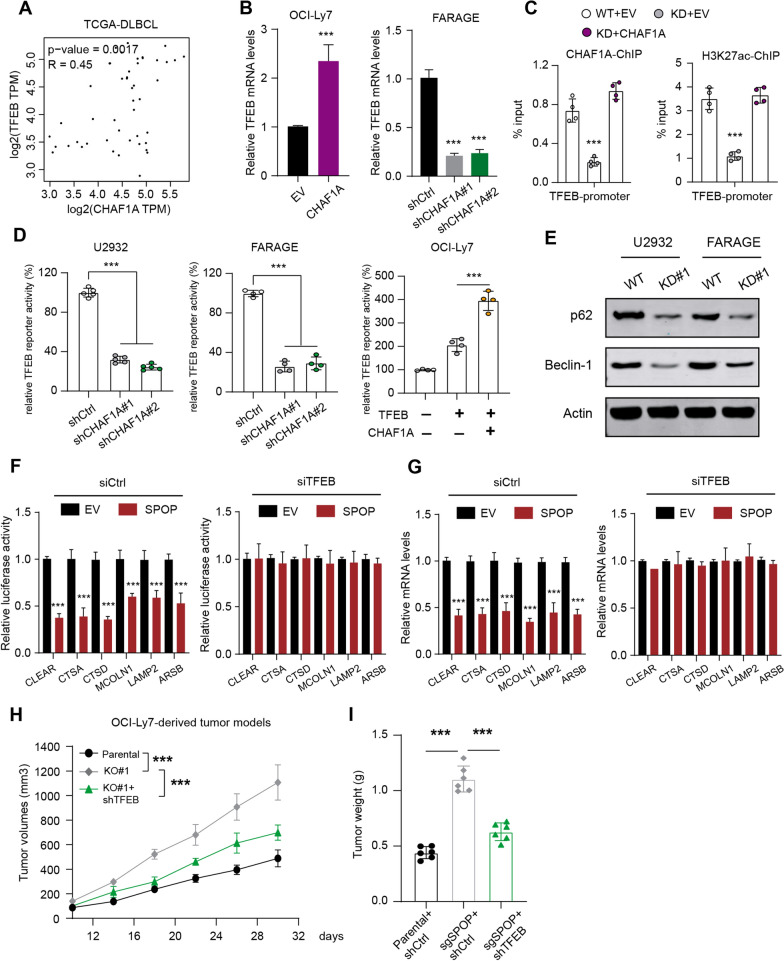
Fig. 5.
SPOP-CHAF1A axis controls tumor autophagy of DLBCL in a TFEB-dependent manner. A Positive associations between CHAF1A and TFEB expressions based on TCGA-DLBCL samples. B The RT-qPCR assay showed that CHAF1A OE could promote TFEB expression (left), while CHAF1A KD reduced TFEB mRNA levels (right panel). C The ChIP-qPCR of H3K27ac markers and CHAF1A in promoter of TFEB gene in U2932 cells, as indicated (N = 4). D The RT-qPCR assay showed that CHAF1A KD could suppress TFEB transcriptional activity in U2932 cells (left panel) and FARAGE cells (middle panel). Relative luciferase activities were normalized versus control. The OCI-Ly7 cells were transfected with TFEB reporters together with TFEB vectors alone or TFEB + CHAF1A plasmids to show the regulation of TFEB reporter activity by CHAF1A (right panel). E Western blotting assay showed that CHAF1A-KD decreased the levels of autophagy-related markers (p62, and Beclin-1) in CHAF1A-silenced U2932 and FARAGE cells relative to control cells. F-G The luciferase activity (F) and mRNA levels (G) of lysosomal genes were measured in control and SPOP-OE OCI-Ly7 cells transfected with siCtrl or siTFEB, individually. H The tumor growth curves were obtained from OCI-Ly7-derived tumor models to show that targeting TFEB significantly suppressed the enhanced tumor growth induced by SPOP deficiency. I The tumor weight of mice from indicated groups was shown and compared. Experiments were performed in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001