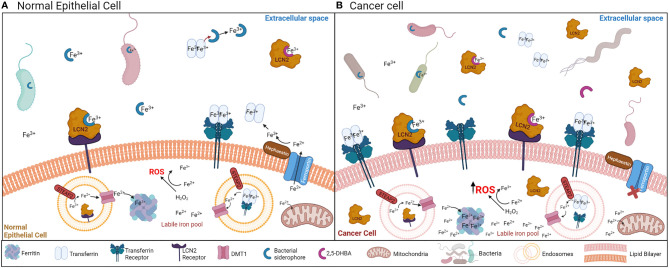
Figure 1.
(A) Mechanisms of iron acquisition between bacteria and normal epithelial cells. Bacteria-secreted siderophores and mammalian siderophores (2,5-DHBA) acquire ferric iron (Fe3+) for bacteria or host uptake. Siderophores can also chelate iron away from transferrin. LCN2 can bind the bacteria-secreted siderophores to sequester ferric iron (Fe3+) away from bacteria. Transferrin and LCN2 bind ferric iron in the extracellular space, and by binding the transferrin receptor or the LCN2 receptor (respectively) in the cell surface, the transferrin/LCN2-iron complex enters the cell through endocytosis. Once iron is in the cytoplasm, it is converted to the ferrous form (Fe2+) by the STEAP2 enzyme, and exits the endosome through the DMT1 transporter. In the cytosol, iron can be stored in ferritin back in the ferric form (Fe3+). Iron can exit the cell via ferroportin, which is regulated by hephaestin. This process is tightly regulated to avoid the generation of ROS from the labile iron pool (free ferrous iron (Fe2+) in the cytoplasm). (B) Mechanisms of iron acquisition between bacteria and cancer cells. Many cancers are characterized by increased bacterial growth and dysbiosis. Iron uptake is increased in cancer cells, which is accomplished by increasing the function of transferrin, the transferrin receptor, ferritin iron storage, and decreasing the function of ferroportin. LCN2 and its receptor are also increased during cancer. Increased ferrous iron (Fe2+) accumulation in the cytosol generates ROS.