Abstract
Significance.
A rigorously designed and calibrated symptom questionnaire for childhood intermittent exotropia would be useful for clinical care and for research.
Purpose.
To Rasch-calibrate and evaluate the previously developed Child Intermittent Exotropia Symptom Questionnaire using data gathered as part of a randomized clinical trial.
Methods.
The questionnaire was administered to 386 children ages 3 to 10 years old with Intermittent Exotropia who were enrolled in a randomized clinical trial comparing overminus to non-overminus spectacles. Participants were followed at 6 and 12 months while on treatment and at 18 months off treatment. Factor analysis determined dimensionality and Rasch analysis evaluated questionnaire performance. Logit values were converted to 0 (best) to 100 (worst). We evaluated differences in questionnaire scores between treatment groups and time points, and correlations with control scores.
Results.
The Child Intermittent Exotropia Symptom Questionnaire was unidimensional. Rasch analysis indicated there was no notable local dependence and no significant differential item functioning for sex or age. There was suboptimal targeting (mean logit −1.62) and person separation was somewhat poor (0.95). There were no significant differences in the Child Intermittent Exotropia Symptom score between overminus spectacles and non-overminus spectacles at 6, 12, and 18 months. Combining data from both treatment groups, there was significant improvement from baseline at all follow up visits (for example, mean change from baseline to 12 months: −6.6 points; 95% CI: −8.6 to −4.6). Child Intermittent Exotropia Symptom scores were not correlated with distance or near control scores at 12 months.
Conclusions.
The 7-item Rasch-scored Child Intermittent Exotropia Symptom Questionnaire is limited by suboptimal performance. Future study is needed to determine whether it may be useful for clinical practice and for research.
Patient-reported outcome measures are used in the assessment of health conditions and response to treatment.1, 2 For childhood health conditions, Patient-reported outcome measures may be directed at the child or the parent (by proxy, to obtain the parent’s perception of the child’s experience; distinct from the child’s own perception of their experience). An initial 22-item symptom questionnaire had been previously developed for childhood intermittent exotropia,3 based on interviews of children with intermittent exotropia and their parents.4 The questionnaire had been reduced to 7 items by selecting items that were associated with reduced health-related quality of life.3 The purpose of the present study was to Rasch calibrate and evaluate the performance of the 7-item Child Intermittent Exotropia Symptom Questionnaire, using data gathered as part of a randomized clinical trial comparing overminus and non-overminus spectacles in childhood intermittent exotropia.5
METHODS
Child Intermittent Exotropia Symptom Questionnaire
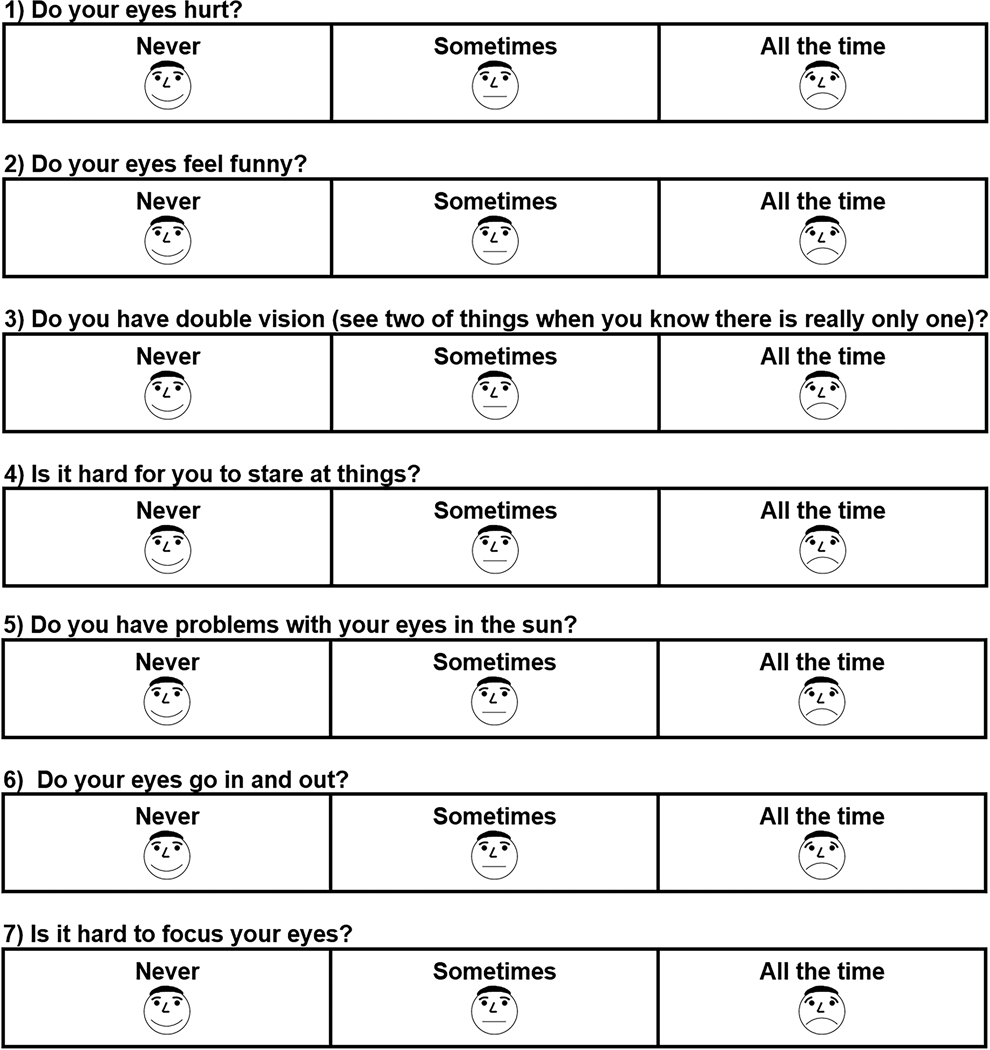
In the present study, the 7-item questionnaire, with 3 response options (“never,” “sometimes,” “all the time,” Figure 1), was administered to young children (3 to <7 years old) by study personnel or self-administered by children 7 to 10 years old, with assistance from study personnel if needed.
Figure 1.
Child Intermittent Exotropia Symptom Questionnaire. The 7-item questionnaire was administered to children ages 3 to <7 years by study personnel or self-administered by children 7 to 10 years old, with assistance from study personnel if needed. Response options for each question were “never,” “sometimes,” and “all the time.”
Data Collection
The randomized trial,5 from which the current data were derived, was funded by the National Eye Institute of the National Institutes of Health and conducted according to the tenets of the Declaration of Helsinki by the Pediatric Eye Disease Investigator Group at 56 clinical sites. The study protocol and Health Insurance Portability and Accountability Act (HIPAA)-compliant informed consent forms were approved by the respective institutional review boards, and a parent or guardian (subsequently referred to as parent) of each participant gave written consent, and children 7 years old and older gave written assent. The study is listed on www.clinicaltrials.gov (NCT02807350, accessed 09/28/2021) and the full protocol is available at www.pedig.net (accessed 09/28/2021).
In the randomized trial, 386 children, aged 3 to <11 years (243 aged 3 to <7 years and 143 aged 7 to <11 years) with intermittent exotropia, were enrolled and randomly assigned 1:1 to either overminus (−2.50D) or non-overminus spectacles.5 Follow-up exams occurred at 6 months and 12 months (in study spectacles; on treatment) and at 18 months (after reducing over minus at 12 months and discontinuing at 15 months).
Statistical Methods
Factor analysis was performed to determine dimensionality of the Child Intermittent Exotropia Symptom Questionnaire by evaluating eigenvalues (eigenvalue >1 indicating multiple factors), and factor loadings. The factor analysis was stratified by follow-up visit to allow for a potential time effect.
Rasch analysis was performed using the Andrich rating-scale model to evaluate questionnaire performance and obtain logit values that were then converted to a 0 (no symptoms) to 100 (worst symptoms) scale. Rasch analysis included confirmation of unidimensionality by principal component analysis of residuals (desired eigenvalue <2), response ordering, local item dependence (desired value <0.6),6 differential item functioning by age (3 to <7 years vs 7 to <11 years and sex (male vs female) (desired contrast <1 logit), item misfit (desired range of mean-square 0.7 to 1.3), targeting of the questionnaire to the sample (desired range −1 to 1 logit), and person separation index (desired value >2) as described in previous studies.4, 7–9 Data were combined from the enrollment and follow-up visits for these Rasch analyses.
We evaluated whether there were treatment group differences (between overminus and non-overminus) in Child Intermittent Exotropia Questionnaire scores, at 6, 12 and 18 months, using analysis of covariance adjusted for the baseline Child Intermittent Exotropia Questionnaire score, and evaluated changes from baseline using the paired t-test (both treatment groups were combined for this analysis because no difference was found between treatment groups). The effect sizes of the changes were calculated by dividing the mean change from baseline at each time point by the standard deviation of the change. For ease in interpretation, summary statistics for group differences and changes, such as means, medians, mean changes from baseline, and 95% CIs, were calculated for the 0 to 100 scores. The p-values for the treatment group differences at 6, 12, and 18 months were calculated for the logit scores and adjusted to control the false discovery rate at 5%.10
Spearman rank correlation coefficients (rs) and 95% confidence intervals (CIs) were calculated at each visit between the Child Intermittent Exotropia Symptom Questionnaire logit score and the exotropia control score (0–5 scale, where 0 is pure phoria and 5 constant tropia)11 rated separately for distance and near. The exotropia control score was the mean of three assessments obtained over the course of an office visit.5, 12 Scatterplots were also created to display those relationships.
No imputation was performed for missing data. Rasch analysis was performed using Winsteps version 4.2.0 (Winsteps Software Technologies, Seattle, WA); all the other analyses were conducted using SAS version 9.4 (SAS Institute Inc. Cary, NC).
RESULTS
Questionnaire Performance
The Child Intermittent Exotropia Symptom Questionnaire was found to be unidimensional by factor analysis (at each of the study time points, 6, 12 and 18 months). Unidimensionality was re-confirmed by principal component analysis (see Appendix Table A1, available at http://links.lww.com/OPX/A566).
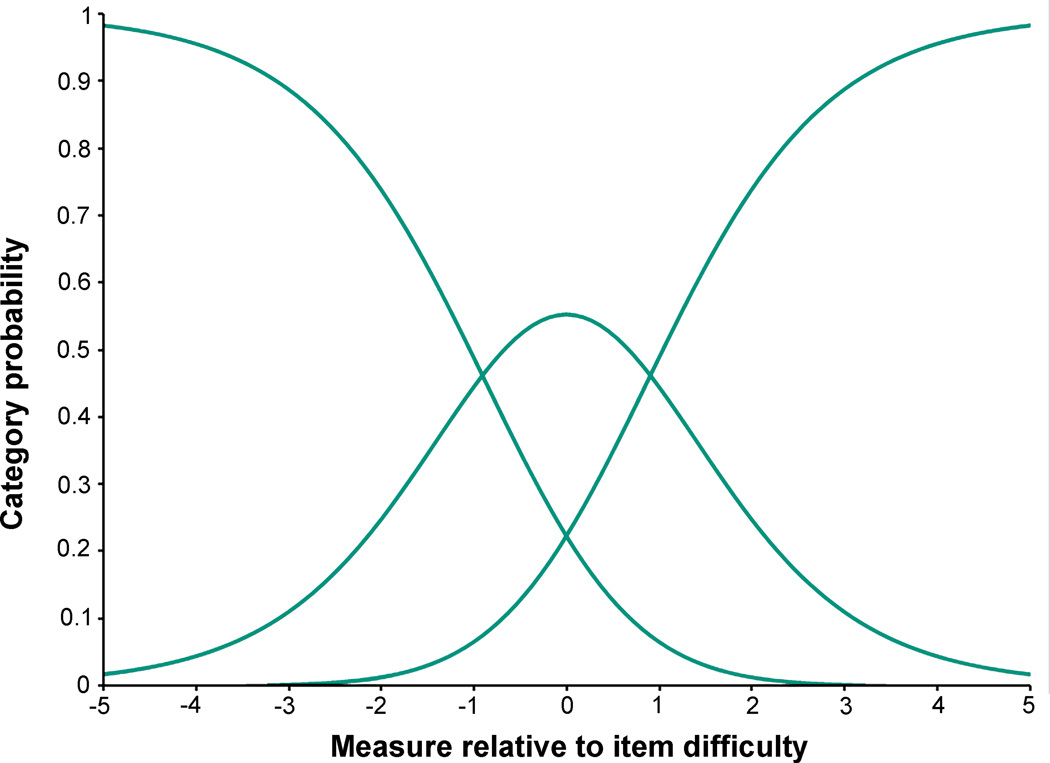
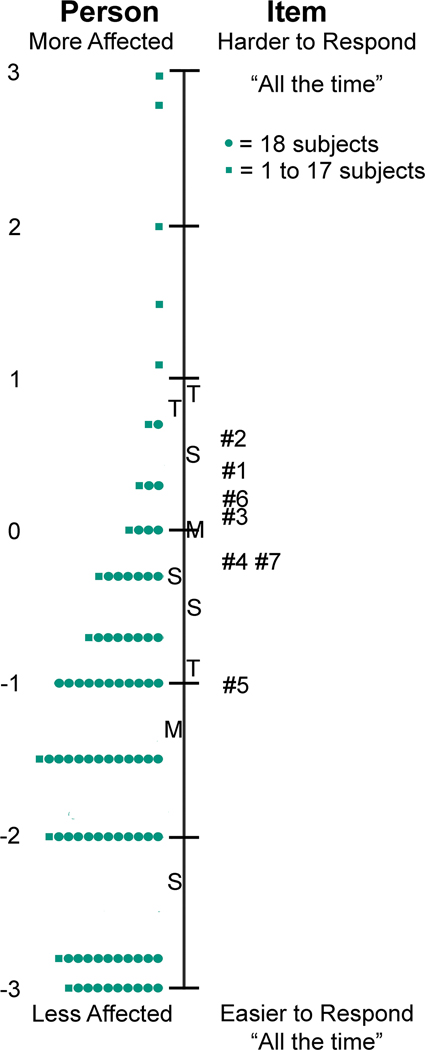
By Rasch analysis, response ordering was appropriate for the 3-response Child Intermittent Exotropia Symptom Questionnaire (Figure 2). There was no notable local dependence (see Appendix Table A2, available at http://links.lww.com/OPX/A566), and no significant differential item functioning for sex or age. The level of misfit was acceptable (see Appendix Table A3, available at http://links.lww.com/OPX/A566). There was suboptimal targeting (mean −1.62 logits; [Figure 3]) and person separation was somewhat poor (0.95).
Figure 2.
Category Thresholds / Category Probability Curves. Category threshold / category probability curves illustrate proper ordering of response categories and utilization of response options.
Figure 3.
Targeting of the Child Intermittent Exotropia (IXT) Symptom Questionnaire. Targeting was suboptimal for the Child IXT Symptom Questionnaire, noting position of mean (M) of respondents on left of figure and questions on right of figure (mean difference −1.62 logits).
Difference in Symptom Score between Overminus and Non-overminus Spectacle Treatment
Comparing children in overminus spectacles with those in non-overminus spectacles, we found no significant differences in Child Intermittent Exotropia Symptom Questionnaire scores at 6 or 12 months (on treatment), or at 18 months (after treatment had been weaned) (Table 1). Because there were no treatment group differences at each time point, the data from both treatment groups were combined for all subsequent analyses.
Table 1.
Child IXT Symptom Questionnaire Scores by Treatment Group.
| Baseline Visit | 6-Month Visit | 12-Month Visit | 18-Month Visit | |||||
|---|---|---|---|---|---|---|---|---|
| Overminus | Non-overminus | Overminus | Non-overminus | Overminus | Non-overminus | Overminus | Non-overminus | |
| Child IXT Symptoms Score | N = 181 | N = 183 | N = 182 | N = 167 | N = 180 | N = 165 | N = 174 | N = 153 |
| Mean (SD) | 34.5 (17.2) | 35.3 (17.4) | 28.8 (16.9) | 31.1 (16.6) | 28.4 (15.3) | 28.1 (16.2) | 28.6 (15.3) | 28.8 (16.4) |
| Median (IQR) |
37.4 (25.7, 46.1) |
37.4 (25.7, 46.1) |
32.2 (15.7, 37.4) |
32.2 (15.7, 42.0) |
32.2 (15.7, 39.7) |
32.2 (15.7, 37.4) |
32.2 (15.7, 37.4) |
32.2 (15.7, 42.0) |
| Range | 0 to 99.9 | 0 to 99.9 | 0 to 99.9 | 0 to 68.1 | 0 to 62.9 | 0 to 74.5 | 0 to 58.4 | 0 to 74.5 |
| Difference in means (95% CI)a | n/ab | −3.05 (−6.18, 0.09) | 0.24 (−2.93, 3.42) | −0.45 (−3.95, 3.04) | ||||
| P-valuea | n/ab | 0.17 | 0.88 | 0.88 | ||||
| Change from Baseline | N = 172 | N = 163 | N = 171 | N = 161 | N = 162 | N = 148 | ||
| Mean (SD) | n/a | n/a | −6.5 (16.7) | −4.0 (18.9) | −6.2 (17.3) | −7.1 (20.5) | −5.9 (20.8) | −5.6 (20.9) |
IXT = intermittent exotropia; SD = standard deviation; IQR = interquartile range; CI = confidence interval
Treatment group differences (mean and 95% CI) in Rasch 0–100 scores were estimated at 6, 12, and 18 months using an ANCOVA model, adjusted for the baseline value of the respective score. P-values were calculated by running the same models on the Logit scores. P-values were adjusted to control the false discovery rate at a probability level of 5%.
Statistical comparison of scores was not done at baseline.
Change in Symptom Scores from Baseline
In a combined cohort with both treatment groups, Child Intermittent Exotropia Symptom scores all showed significant improvement from baseline to 6-, 12-, and 18-months (Table 2).
Table 2.
Child IXT Symptom Questionnaire Scores Over Time with treatment groups combined.
| Baseline Visit | 6-Month Visit | 12-Month Visit | 18-Month Visit | |
|---|---|---|---|---|
| Child IXT Symptom Score | N = 364 | N = 349 | N = 345 | N = 327 |
| Mean (SD) | 34.9 (17.2) | 29.9 (16.8) | 28.3 (15.7) | 28.7 (15.8) |
| Median (IQR) |
37.4 (25.7, 46.1) |
32.2 (15.7, 42.0) |
32.2 (15.7, 37.4) |
32.2 (15.7, 37.4) |
| Range | 0 to 99.9 | 0 to 99.9 | 0 to 74.5 | 0 to 74.5 |
| Change from Baseline | N = 335 | N = 332 | N = 310 | |
| Mean (SD)a | n/a | −5.3 (17.8) | −6.6 (18.9) | −5.7 (20.8) |
| 95% CI for Mean Changea | n/a | (−7.2, −3.4) | (−8.6, −4.6) | (−8.1, −3.4) |
| Effect Sizeb | n/a | 0.30 | 0.35 | 0.28 |
IXT = intermittent exotropia; SD = standard deviation; IQR = interquartile range; CI = confidence interval
The paired t-test was used to estimate mean changes from baseline to follow-up visits and the 95% CIs for the 0–100 scores. P-values were not reported.
This is the absolute value of the effect size for the overall improvement from baseline. First, the 0–100 score at baseline was subtracted from the 0–100 score at each follow-up visit for each participant. Then, this change from baseline was averaged across all participants; this average was divided by the standard deviation of the changes to calculate the effect size.
Relationship of Child Intermittent Exotropia Symptom Score to Exotropia Control Scores
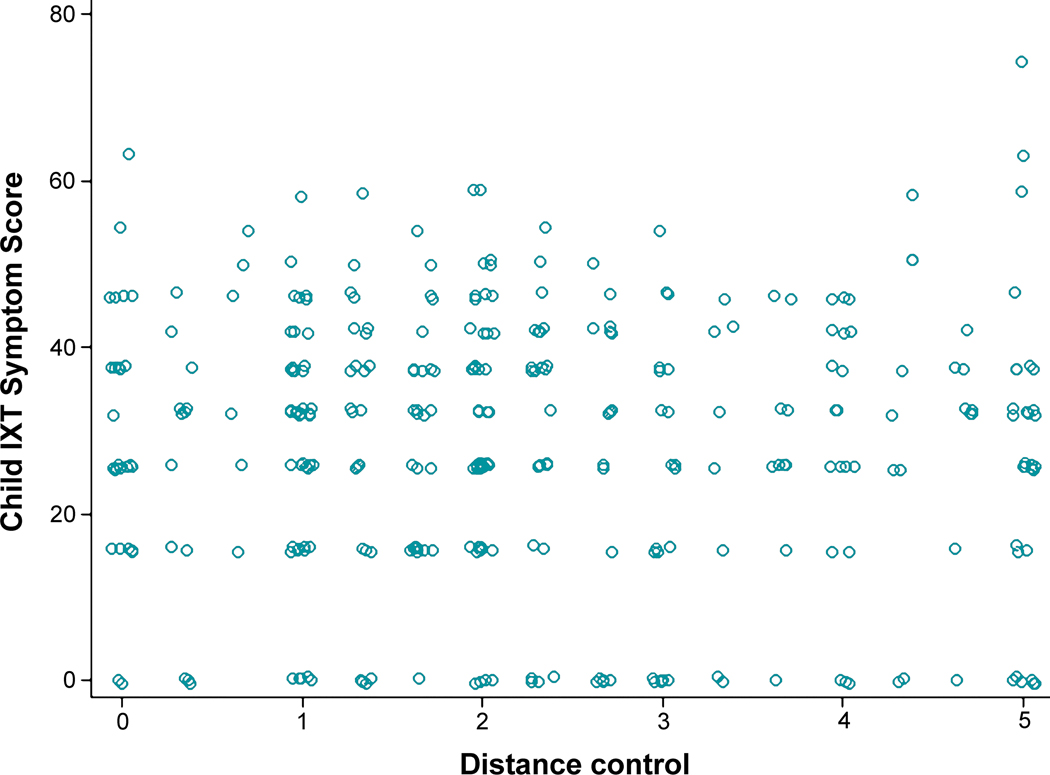
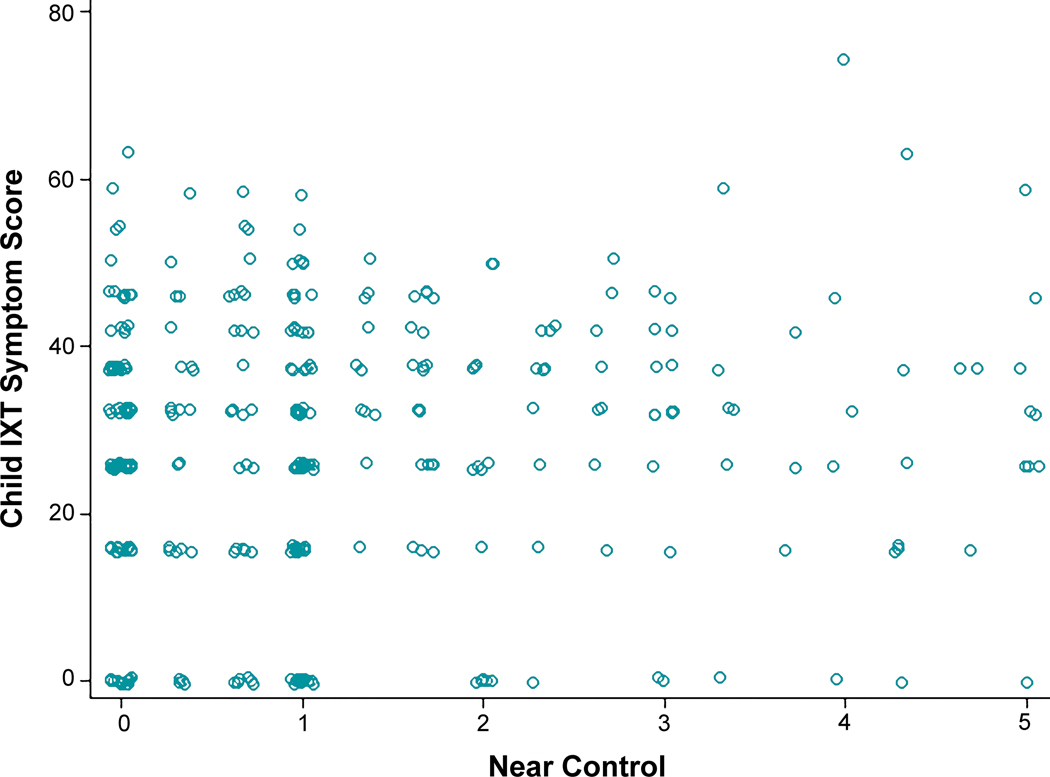
Child Intermittent Exotropia Symptom scores were not correlated with distance or near control scores at 6 months (rs = −0.01, 95% CI −0.12 to +0.09, and rs = 0.002, 95% CI −0.10 to +0.11), 12 months (rs = −0.04, 95% CI −0.15 to +0.07, and rs = 0.08, 95% CI −0.03 to +0.18) (Figure 4 and Figure 5), or 18 months (rs = 0.04, 95% CI −0.07 to +0.15, and rs = 0.15, 95% CI 0.05 to +0.26).
Figure 4.
Relationship of Child Intermittent Exotropia (IXT) Symptom Score with Distance Control at 12 Months. Scatter plot of distance control at 12 months versus child IXT symptom score at 12 months. The Spearman correlation coefficient, calculated on the logit scale, was −0.04 (95% CI −0.15, 0.07), indicating no correlation; results were similar at all other visits.
Figure 5.
Relationship of Child Intermittent Exotropia (IXT) Symptom Score with Near Control at 12 Months.Scatter plot of near control at 12 months versus child IXT symptom score at 12 months. The Spearman correlation coefficient, calculated on the logit scale, was 0.08 (95% CI −0.03, 0.18), indicating no correlation; results were similar for other visits.
DISCUSSION
We have developed a 7-item Rasch-calibrated questionnaire for the assessment of child self-reported intermittent exotropia symptoms (Child Intermittent Exotropia Symptom Questionnaire). The final questionnaire had acceptable psychometric performance and was able to detect changes in child intermittent exotropia symptoms over time, but did not detect a difference between overminus and non-overminus spectacle treatment for intermittent exotropia.
We are unaware of other questionnaires designed to evaluate the presence and severity of symptoms in intermittent exotropia. Previous child questionnaires for intermittent exotropia have been designed to evaluate health-related quality of life associated with intermittent exotropia 4, 7 whereas the Child Intermittent Exotropia Symptom Questionnaire specifically evaluates symptoms. Patient-reported outcome measures, such as the Child Intermittent Exotropia Symptom Questionnaire, provide a standardized means of detecting symptoms and scoring their frequency. In addition, the Child Intermittent Exotropia Symptom Questionnaire can be easily incorporated into clinical research (questionnaire and Rasch scoring look-up tables are freely available at www.pedig.net).
A strength of the Child Intermittent Exotropia Symptom Questionnaire is that the items were derived from interviews with children with intermittent exotropia and their parents.4 Deriving questionnaire items directly from individuals with the condition of interest increases the likelihood of evaluating concerns that are important to that specific population. In the present study, factor analysis and Rasch analysis confirmed the unidimensionality of the Child Intermittent Exotropia Symptom Questionnaire and provided Rasch-calibrated scoring. We had used stepwise multiple variable regression analysis to select items that were associated with reduced health related quality of life in any of the IXTQ domains.3 An alternative method of development would have been to administer the complete 22-item questionnaire to a large cohort of children with intermittent exotropia and perform the previously described9 steps of binning, winnowing, and de novo Rasch analysis, but this was not the method used for this particular questionnaire.3
We did not find a significant difference in Child Intermittent Exotropia Symptom Questionnaire scores between the overminus and non-overminus groups at any of the follow-up visits. This finding is likely a reflection of the modest improvement of intermittent exotropia control from the overminus lenses at 6 months and 12 months (on-treatment) and lack of treatment effect at 18 months (off treatment).5 The mean difference in control scores was 0.8 points at 12 months (95% CI +0.5 to +1.0 points, on the 0 to 5 scale), and 0.2 points (95% CI −0.04 to +0.5) at 18 months.5 The Child Intermittent Exotropia Symptom Questionnaire may not have been sensitive enough to detect a treatment group difference at 12 months, when the mean control scores were significantly different between groups.5 It is also possible that overminus treatment may have been insufficient to relieve symptoms.
An alternative explanation for the failure to find a significant difference in intermittent exotropia symptoms between overminus and non-overminus treatment for intermittent exotropia is that children may not experience more frequent symptoms when there is worse control of intermittent exotropia. Indeed, we found no correlation between the Child Intermittent Exotropia Symptom Questionnaire score and the distance or near control score (Figures 4 and 5). It is also possible that some symptoms such as “do your eyes hurt” might be associated with good intermittent exotropia control, if the child is exerting a great deal of fusional vergence effort or accommodative effort. Other symptoms, such as “do you find it hard to stare at things,” might be associated with poor intermittent exotropia control if the child is unable to maintain binocular fusion and if binocular staring was considered binocular. When such items are combined, the net symptom questionnaire score might not correlate with the control score. It is also possible that some children with intermittent exotropia have sensory adaptations (for example, suppression or anomalous retinal correspondence) that mitigate subjective symptoms, while other children with intermittent exotropia do not have these adaptations. The relationship between sensory adaptations to strabismus and symptoms is worthy of further study.
An additional reason why we failed to find a difference in symptoms between children undergoing overminus versus non-overminus treatment for intermittent exotropia is the possibility that children may have had a decrease in IXT symptoms with overminus treatment but the effect may have been masked by an increase in symptoms with the overminus treatment itself (such as strain and blur). These potentially counterbalancing effects might have resulted in no net effect. Nevertheless, in the original study, we found no marked differences between responses to spectacle related questions between treatment groups.
We found improvement in the symptom scores from baseline to 6 months, from baseline to 12 months, and from baseline to 18 months in both treatment groups. These findings may reflect regression to the mean; although we did not specify a threshold questionnaire score for eligibility, we did specify an enrollment criterion related to a minimum severity of intermittent exotropia control (a mean distance control score of at least 2 based on the mean of 3 assessments during a single examination). While we did not find a relationship between the intermittent exotropia control score and the Child Intermittent Exotropia Symptom Questionnaire score, it is possible that children with worse symptoms at the time of enrollment were preferentially enrolled and thereby at risk of regression to the mean. Alternatively, improved symptom scores may reflect our previous finding that some clinical aspects of intermittent exotropia may show improvement with time (i.e., control, stereoacuity, and magnitude of deviation) over a 3-year period of observation.13 There is also a possibility of a placebo effect of glasses wear, because all participants received glasses and may have thought they were receiving overminus treatment.
There are several limitations to the present study in addition to those already described. Personnel administering the questionnaire were not masked to the participant’s treatment. The Rasch-calibrated Child Intermittent Exotropia Symptom Questionnaire did not have the desired targeting or person separation indices, and so may have been insensitive to true differences between treatment groups, if they existed. Nevertheless, the problems of less than desired targeting, and less than desired person separation, are often found for questionnaires designed for children, for example the PedEyeQ.9 Such suboptimal performance indices would render pediatric questionnaires less sensitive than questionnaires designed for adults. It is also possible that if we had more data using the original 22 item version of the questionnaire, and if we had selected the best items using psychometric methods, rather than logistic regression, we might had developed a short questionnaire that may have had better targeting and measurement precision. Poorer than desired questionnaire performance was also likely when the items were not optimally separated on the person-item map (Figure 3). It is possible that person separation might have been improved by a greater number of response categories, but children younger than 7 years of age typically are unable to successfully use more than 3 response categories.
In the present study we have Rasch calibrated a previously developed 7-item patient-reported outcome measure to evaluate child-reported symptoms in intermittent exotropia (the Child intermittent exotropia Symptom Questionnaire). The Child Intermittent Exotropia Symptom Questionnaire appears limited by suboptimal performance. Future study is needed to determine whether it may be useful for clinical practice and for research.
ACKNOWLEDGMENTS
Amra Hercinovic had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Research reported in this publication was supported by the National Eye Institute of the National Institutes of Health, under Award Numbers EY011751, EY023198, EY018810 and EY024333. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This manuscript was accepted as a poster presentation as part of the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting (May 2021, Virtual Meeting).
APPENDICES
Appendix Table A1.
Dimensionality Analysis of the Child IXT Symptom Questionnaire
| Child IXT Symptoms (7 items) | ||
|---|---|---|
| Eigen | % | |
| Total raw variance | 10.0 | 100 |
| Explained by measures | 3.0 | 30 |
| Explained by persons | 1.3 | 13 |
| Explained by items | 1.8 | 18 |
| Total unexplained | 7.0 | 70 |
| 1st contrast | 1.4 | 14 |
| 2nd contrast | 1.2 | 12 |
| 3rd contrast | 1.2 | 12 |
| 4th contrast | 1.1 | 11 |
| 5th contrast | 1.1 | 11 |
| Item Number | 1st Contrast Loading | |
| 1 | 0.23 | |
| 2 | 0.17 | |
| 3 | −0.30 | |
| 4 | −0.61 | |
| 5 | 0.83 | |
| 6 | −0.13 | |
| 7 | −0.35 | |
Appendix Table A2.
Local Dependence Analysis of the Child IXT Symptom Questionnaire
| Child IXT Symptoms (7 items) | ||
|---|---|---|
| Correlation | Item | Item |
| −0.30 | 4 | 5 |
| −0.25 | 3 | 5 |
| −0.23 | 5 | 6 |
| −0.21 | 5 | 7 |
| −0.20 | 1 | 7 |
| −0.18 | 3 | 7 |
| −0.18 | 4 | 6 |
| −0.17 | 2 | 4 |
| −0.17 | 6 | 7 |
| −0.17 | 1 | 6 |
| −0.16 | 2 | 3 |
| −0.15 | 1 | 3 |
| −0.14 | 2 | 5 |
| −0.14 | 3 | 4 |
| −0.13 | 2 | 7 |
| −0.13 | 1 | 5 |
| −0.13 | 1 | 4 |
| −0.12 | 1 | 2 |
| −0.12 | 3 | 6 |
| −0.10 | 2 | 6 |
Appendix Table A3.
Infit and Outfit Errors of the Child IXT Symptom Questionnaire.
| Child IXT Symptoms | ||||
|---|---|---|---|---|
| Item | Infit | Outfit | ||
| Mean Square | Standard Z-score | Mean Square | Standard Z-score | |
| 1 | 0.91 | −2.2 | 0.94 | −1.1 |
| 2 | 0.97 | −0.7 | 0.90 | −1.7 |
| 3 | 1.02 | 0.4 | 1.07 | 1.4 |
| 4 | 0.98 | −0.4 | 0.94 | −1.5 |
| 5 | 1.15 | 4.2 | 1.14 | 3.6 |
| 6 | 1.03 | 0.8 | 1.07 | 1.3 |
| 7 | 0.89 | −3.1 | 0.85 | −3.6 |
REFERENCES
- 1.Braithwaite T, Calvert M, Gray A, et al. The Use of Patient-Reported Outcome Research in Modern Ophthalmology: Impact on Clinical Trials and Routine Clinical Practice. Patient Relat Outcome Meas 2019;10:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims: Draft Guidance. Health Qual Life Outcomes 2006;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatt SR, Leske DA, Liebermann L, Holmes JM. Symptoms in Children with Intermittent Exotropia and Their Impact on Health-Related Quality of Life. Strabismus 2016;24:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatt SR, Leske DA, Yamada T, et al. Development and Initial Validation of Quality-of-Life Questionnaires for Intermittent Exotropia. Ophthalmology 2010;117:163–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen AM, Erzurum SA, Chandler DL, et al. Overminus Lens Therapy for Children 3 to 10 Years of Age with Intermittent Exotropia: A Randomized Clinical Trial. JAMA Ophthalmol 2021;139:464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linacre JM. Winsteps Rasch Measurement Computer Program User’s Guide. Beaverton, OR: Winsteps.com; 2011. [Google Scholar]
- 7.Leske DA, Holmes JM, Melia M, on behalf of Pediatric Eye Disease Investigator Group. Evaluation of the Intermittent Exotropia Questionnaire Using Rasch Analysis. JAMA Ophthalmol 2015;133:461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leske DA, Hatt SR, Liebermann L, Holmes JM. Evaluation of the Adult Strabismus-20 (AS-20) Questionnaire Using Rasch Analysis. Invest Ophthalmol Vis Sci 2012;53:2630–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatt SR, Leske DA, Castañeda YS, et al. Development of Pediatric Eye Questionnaires for Children with Eye Conditions. Am J Ophthalmol 2019;200:201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Statist Soc (B) 1995;57:289–300. [Google Scholar]
- 11.Mohney BG, Holmes JM. An Office-Based Scale for Assessing Control in Intermittent Exotropia. Strabismus 2006;14:147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatt SR, Liebermann L, Leske DA, et al. Improved Assessment of Control in Intermittent Exotropia Using Multiple Measures. Am J Ophthalmol 2011;152:872–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pediatric Eye Disease Investigator Group. Three-Year Observation of Children 3 to 10 Years of Age with Untreated Intermittent Exotropia. Ophthalmology 2019;126:1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]