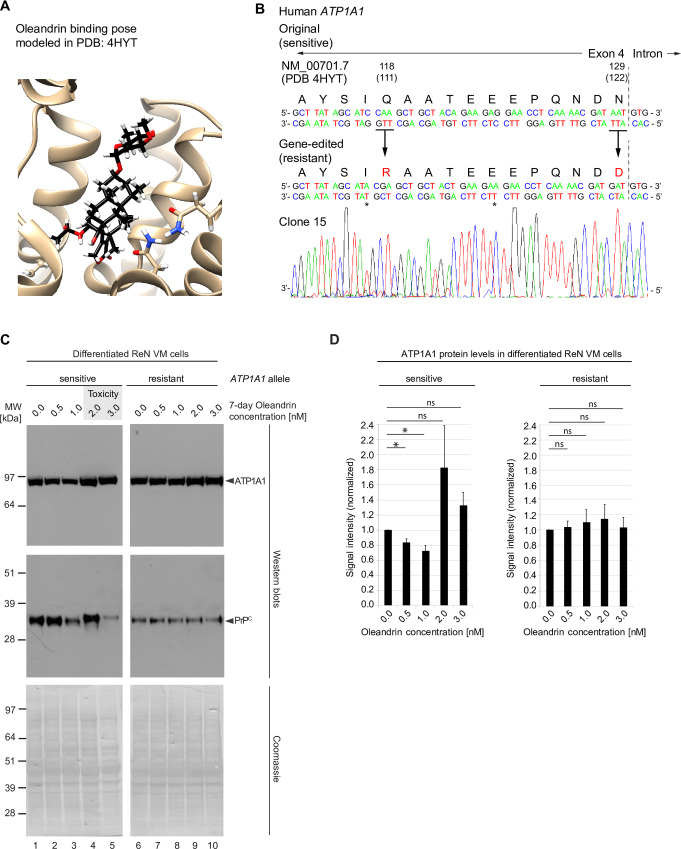
Fig 6. CG-dependent reduction of PrPC levels depends on CG-ATP1A1 ligand-receptor engagement.
(A) Predicted oleandrin binding pose modeled in ATP1A1 structure reported in PDB entry 4HYT. (B) Genetic sequencing results confirm successful amino acid substitutions Q118R and N129D, two changes required to render the human ATP1A1 resistant to CGs, in a ReN VM clone. (C) Comparison of ATP1A1 levels (top panel) and PrPC levels (bottom panel) in response to oleandrin treatment in ReN VM cells that express either the CG sensitive wild-type or the CRISPR-Cas9-engineered CG resistant ATP1A1 alleles. In wild-type cells levels of ATP1A1 and PrPC decreased as oleandrin concentration increased from 0 to 1 nM. When oleandrin was administered at a concentration of 2 nM, levels of ATP1A1 and PrPC rebounded, and toxicity was observed (grey shaded box). In contrast, steady-state protein levels of ATP1A1 and PrPC remained stable at all oleandrin concentrations tested in ReN VM cells expressing the resistant form of ATP1A1, and no toxicity was observed. Coomassie staining documents that protein loading was adjusted across lanes. (D) Graphs depicting quantitation of steady-state levels of ATP1A1 in the presence of oleandrin concentrations up to 3 nM in CG-sensitive wild-type and CG-resistant ReN VM cells.