Figure 2.
Mitochondrial morphological change by accumulated Drp1 and dysfunction in heart-specific MITOL-KO mice
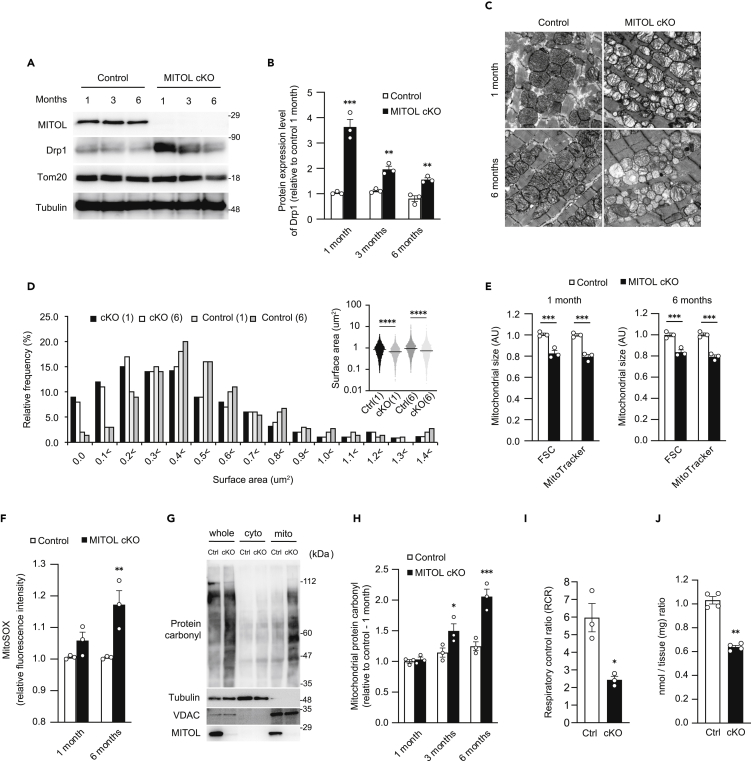
(A and B) Heart-specific MITOL KO enhances Drp1 accumulation in the heart. Samples were collected from the hearts of MITOLflox/flox (Control) and MITOLflox/flox;αMHC-CreMer (MITOL cKO) mice treated with tamoxifen for the indicated periods, followed by immunoblotting with indicated antibodies. The relative protein levels of Drp1 were quantified by densitometry. Data are standardized to tubulin levels and are expressed relative to control mice. Mean ±SEM (n = 3). Analysis was performed with two-way ANOVA followed by Bonferroni post hoc analysis. ∗∗∗p < 0.001, ∗∗p < 0.01.
(C and D) Abnormal morphologies of mitochondria in MITOL-KO mouse cardiomyocytes. Representative electron microscopic images of mitochondria from the hearts of MITOLflox/flox (Control) and MITOLflox/flox;αMHC-CreMer (MITOL cKO) mice treated with tamoxifen for the indicated periods. 27,600-fold magnification (C). Mitochondrial size was represented as median surface area, and frequency distributions of mitochondrial surface were calculated from mitochondria imaged by TEM (D). Kruskal-Wallis test, ∗∗∗∗p < 0.0001. Data are median values. Bar, 500 nm.
(E) Decreased size of mitochondria in MITOL-KO mouse cardiomyocytes. Cardiac mitochondrial areas of MITOLflox/flox (Control), and MITOLflox/flox;αMHC-CreMer (MITOL cKO) mice treated with tamoxifen for the indicated periods were measured. Mitochondrial fractions isolated from cardiomyocytes of MITOLflox/flox and MITOLflox/flox;αMHC-CreMer mice treated with tamoxifen for the indicated periods were stained with MitoTracker, followed by flow cytometric analysis. Bar graphs show the relative levels of mean fluorescence intensity of forward-scatter (FSC) and MitoTracker to MITOLflox/flox mice. Mean ±SEM (n = 3). ∗∗∗p < 0.001.
(F) Mitochondrial ROS was upregulated in MITOL-KO mouse cardiomyocytes. Cardiac mitochondrial fractions of MITOLflox/flox (Control), and MITOLflox/flox;αMHC-CreMer (MITOL cKO) mice treated with tamoxifen for the indicated periods were stained with MitoSOX and mitochondrial-derived superoxide generation was measured by flow cytometric analysis. Bar graph shows the relative levels of mean fluorescence intensity of MitoSOX to MITOLflox/flox mice. Mean ± SEM (n = 3). Analysis was performed with two-way ANOVA followed by Bonferroni post hoc analysis. ∗∗p < 0.01.
(G and H) MITOL knockout induces mitochondrial oxidative damage in cardiomyocytes. Protein carbonyl contents of cardiac mitochondrial extracts were determined by protein carbonyls western blot detection kit (G). The levels of carbonylated proteins were quantified by densitometry. Data are standardized to VDAC levels and are expressed relative to MITOLflox/flox (Ctrl) mice prior to tamoxifen treatment. Mean ±SEM (n = 3). Analysis was performed with two-way ANOVA followed by Bonferroni post hoc analysis. ∗p < 0.05, ∗∗∗p < 0.001. (H).
(I) Reduced oxygen consumption rate of mitochondria isolated from MITOL-KO mouse cardiomyocytes. The respiratory control ratio (RCR) represents the mitochondrial coupling state. Mean ±SEM (n = 3). ∗p < 0.05, Student’s t-test.
(J) Reduced ATP content in the MITOL-KO heart. ATP content was measured by luciferase assay. Mean ±SEM (n = 4). ∗∗p < 0.01, Student’s t-test.