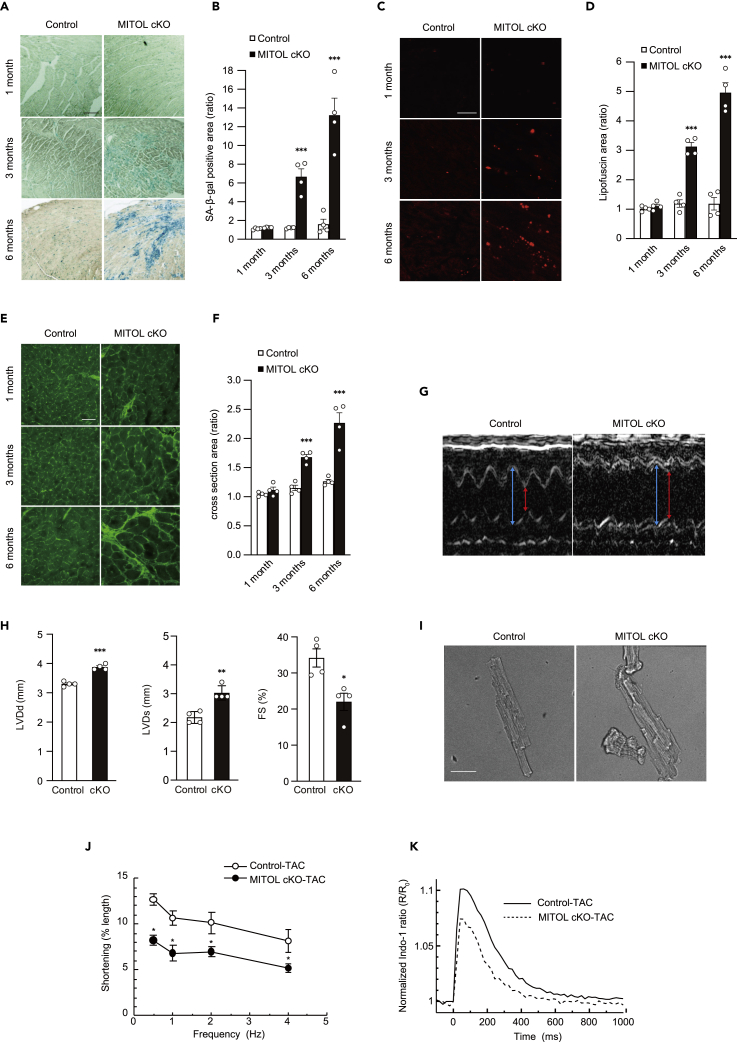
Figure 4.
Cardiac ablation of MITOL caused heart aging and vulnerability to TAC treatment
(A and B) Senescent cardiomyocytes were accumulated in MITOL-cKO mice. Senescent cells were detected by SA-β-Gal staining in the heart for the indicated periods after MITOL deletion by tamoxifen (A). Bar graph shows the percentage of SA-β-Gal positive cells in the heart sections (B). Mean ±SEM (n = 4). ∗∗∗p < 0.001. Bar, 200 μm.
(C and D) Accumulation of lipofuscin and cardiomyocyte hypertrophy in MITOL-cKO mice. Frozen heart tissue sections were analyzed for lipofuscin by fluorescence microscopy. Lipofuscin was detected as autofluorescence (C). The relative content of lipofuscin (area of lipofuscin granules/field of the myocardium) was analyzed with Image J (D). Mean ±SEM (n = 4). ∗∗∗p < 0.001. Bar, 30 μm.
(E and F) Frozen heart tissue sections were also stained with FITC-conjugated wheat germ agglutinin (WGA) to detect cardiomyocyte borders (E). The areas of cardiomyocytes were measured from 100 cells. Mean ±SEM (n = 4). ∗∗∗p < 0.001. Bar, 50 μm. Bar graphs show relative size of cells compared with control one month after tamoxifen-treatment (F).
(G) Representative M-mode echocardiograms from control-TAC and MITOL-cKO-TAC mice. Mice for three months after tamoxifen injection were subjected to TAC surgery for two weeks. Red arrow, LVIDs. Blue arrow, LVIDd.
(H) Impaired systolic function without cardiac hypertrophy in MITOL-cKO-TAC mice. Echocardiographic analysis of left ventricular dimensions and cardiac function in control-TAC and MITOL-cKO-TAC mice. The graph shows the percentage of FS. Mean ±SEM (n = 4). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, Student’s t-test.
(I and J) Representative images of control-TAC and MITOL-cKO-TAC cardiomyocytes (I). Bar, 50 μm. Frequency-dependent shortening of cardiomyocytes isolated from control-TAC and MITOL-cKO-TAC mice (J). Mean ±SEM (n = 4). ∗p < 0.05.
(K) Averaged traces of Ca2+ transients evoked at electric stimulation at 1 Hz. Indo-1 fluorescence ratio (R) was normalized to the averaged ratio before electric stimulation (R0).