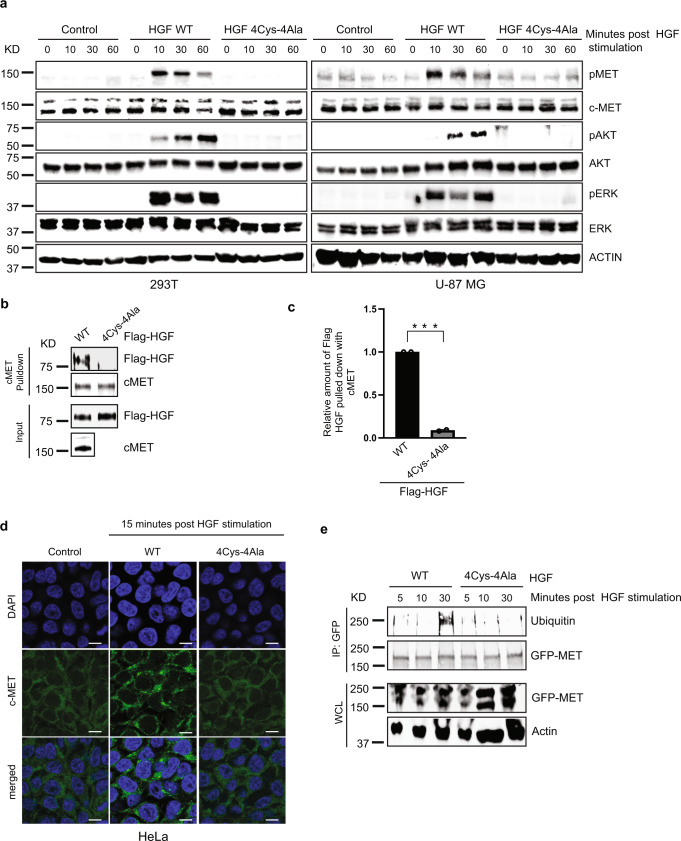
Fig. 2. HGF PAN domain regulates c-MET signaling cascade via four core cysteines.
a Mutation of the core cysteines in HGF PAN domain blocks HGF-induced c-MET signaling. Both 293T and U-87 MG cells were stimulated with HGF WT and HGF 4Cys-4Ala proteins for the indicated amount of time. Cells were harvested and immunoblot analysis shows the absence of phosphorylation for c-MET, AKT, and ERK in presence of HGF 4Cys-4Ala. Representative blot images from n = 2 experiments for individual cell line. b Immunoblot showing that the core cysteines on the HGF PAN domain regulate its binding with c-MET. c-MET was immunoprecipitated from 293T cells on anti-MET bound beads. In-vitro translated Flag-tagged HGF WT and HGF 4Cys-4Ala were added to the beads as indicated to detect the interaction between endogenous c-MET and Flag-HGF WT and Flag-HGF 4Cys-4Ala. c Right panel, quantification of the band intensities (n = 2; *** p < 0.0005 (Student’s t test)). Immunoprecipitated Flag-HGFs band intensities were normalized to the respective c-MET IP bands and then further normalized to HGF-WT. d HGF PAN domain defines perinuclear translocation of c-MET in cells. HeLa cells were stimulated with HGF WT and HGF 4Cys-4Ala proteins for the indicated amount of time following serum starvation. Post stimulation cells were fixed, mounted and endogenous c-MET localization pattern was visualized using Zeiss LSM 710 at 63x objective. Scale bars represent 20 μm. The images shown are representative from three independent biological replicates (average 100 cells were observed for each condition per replicate). e PAN domain regulates c-MET ubiquitination. In vivo ubiquitination assay shows that HGF WT promotes c-MET ubiquitination in a PAN-dependent manner. 293T cells were transfected with the construct c-MET-C-GFPSpark. After serum starvation, cells were stimulated with HGF WT and HGF 4Cys-4Ala as indicated. The lysates were collected at specific time points and incubated with anti-GFP protein G beads. Ubiquitinated-c-MET proteins were eluted, resolved by SDS-PAGE, and immunoblotted with the indicated antibodies.