Figure 3.
Expansion microscopy
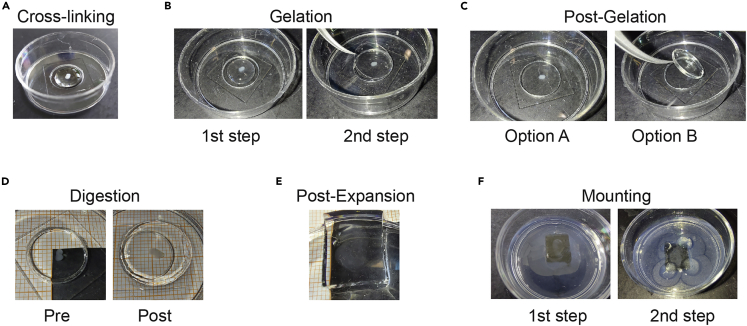
(A) Cross-linking: Transfer each OTC into the microwell of a MatTek dish (step 13) and add 200 μL Acryloyl-X into the microwell (step 14).
(B) Gelation: After 2× 15 min PBS washes, add 200 μL fresh gelling solution in the microwell and let stand at 4°C for 30 min (left panel, steps 15–19); replace the old gelling solution with 150 μL fresh gelling solution, carefully place a coverslip on top and let stand at 37°C for 2 h (right panel, steps 21 and 22).
(C) Post-Gelation: Gently pull off the coverslip (Option A); sometimes the coverslip sticks to the gel, in which case both should be removed from the microwell (Option B; step 23).
(D) Digestion: Add 2 mL digestion buffer and allow the gel to stand overnight (12 h–16 h) at 22°C (left panel; steps 24 and 25). The OTCs are already expanding during the digestion: compare the left (before digestion) and right (after digestion) panels (steps 25 and 26). Note that the gel has expanded out of the microwell.
(E) Expansion: Add ∼ 5 mL distilled or MilliQ water to the dish and let the OTC sit for ∼ 20 min; repeat until expansion reaches a plateau (in our hands, after 5–6 baths). The picture shows the same sample from D, now expanded after the fifth bath (step 28). The gel was cut around the sample to facilitate handling.
(F) Embedding: Add viscous agarose around the sample and wait a few minutes for the agarose to gel (1st step), then add a few extra drops to the edges of the gel to prevent movements in Z (2nd step), being careful not to cover the OTC to avoid diffraction artifacts (steps 32 and 33).