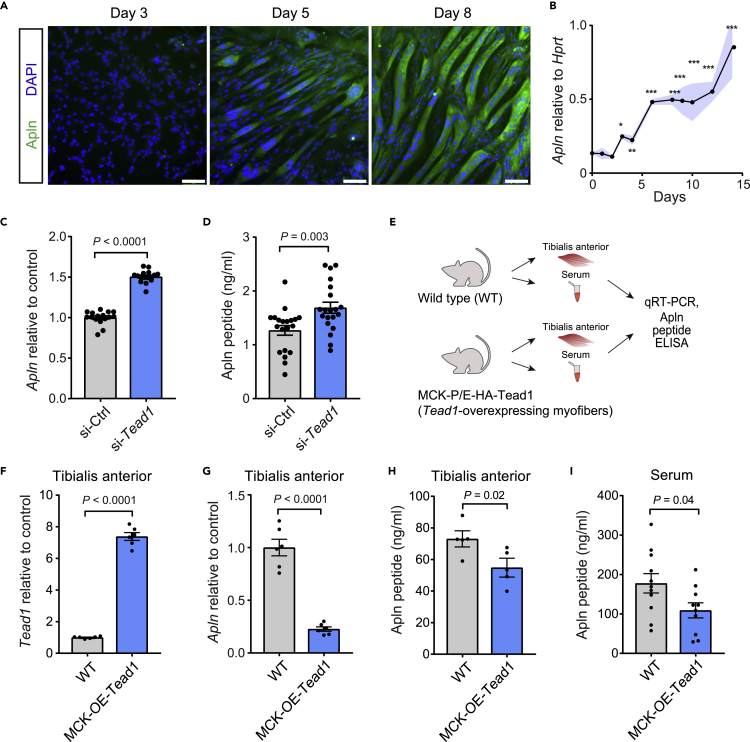
Figure 2.
Apelin is repressed by Tead1 in muscle cells in vitro and in vivo
(A) Immunostaining of Apln protein during C2C12 myotube differentiation. Scale bars, 100 μm.
(B) Quantification of Apln mRNA by RT-qPCR during C2C12 myotube differentiation relative to Hprt using 2–dCt method. n = 4 cell culture replicates per time point.
(C and D) Quantification of apelin mRNA by RT-qPCR (C) or apelin peptide in supernatant by ELISA (D) in C2C12 myoblasts transfected with scrambled control or Tead1 targeted siRNAs for 3days n = 16-20 replicates per condition.
(E–I) Analysis of Tead1 and in adult mice over-expression Tead1 in skeletal muscle myofibers under the muscle creatine kinase (MCK) promoter (MCK-OE-Tead1 mice), compared to WT C57BL6 controls. (F and G) Tead1 mRNA (F) and Apln mRNA (G) expression levels were measured by RT-qPCR and normalized to Hprt in tibialis anterior (TA) muscles. n = 6 mice per condition. (H and I) Apln peptide concentration measured by ELISA in TA muscles (H) or serum (I). n = 5 mice per condition for TA; n = 11 mice per condition for serum. All data are presented as mean ± SEM, and p values are reported from two-tailed, unpaired t-tests between conditions. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.