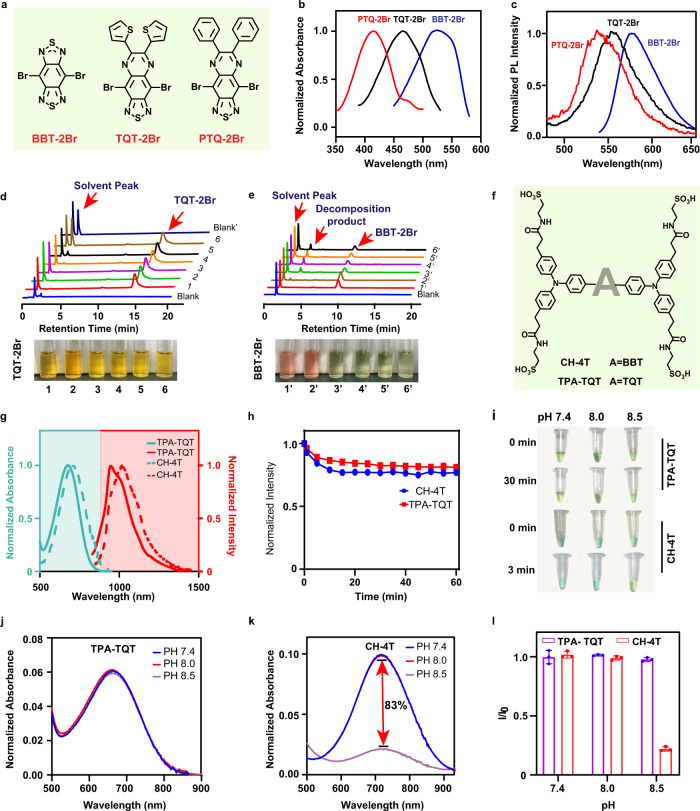
Fig. 2. The comparison of different acceptor NIR-II dyes.
a Chemical structures of BBT-2Br, TQT-2Br, and PTQ-2Br. Absorption (b) and emission (c) spectra of BBT-2Br, TQT-2Br, and PTQ-2Br in dichloromethane. HPLC chromatograms of TQT-2Br (d) and BBT-2Br (e) at various acid-base conditions. Bright-field images of TQT-2Br and BBT-2Br (d, e) in MeOH (5% DMF, 1 mL) at various acid-base conditions. 1,1′: control solution; 2,2′: control solution + excess trifluoroacetic acid (TFA); 3,3′: control solution + 1 µL Triethylamine (TEA); 4,4′: control solution + 5 µL TEA; 5,5′: control solution + 10 µL TEA; 6,6′: control solution + 20 µL TEA; Blank’: 50 µL DMF + 20 µL TEA + 930 µL MeOH. f Chemical structures of TPA-TQT and CH-4T. g The absorption spectra and emission spectra of TPA-TQT and CH-4T in H2O. h Photostability of TPA-TQT and CH-4T under continuous laser irradiation (808 nm, 150 mW cm−2). i Photographs of the TPA-TQT and CH-4T in PBS solutions at various pH values after 808 nm light irradiation. The absorption spectra of j TPA-TQT and k CH-4T in PBS at various pH values under 808 nm laser irradiation (150 mW cm−2). l Plot of I/I0 versus pH. I is the maximal NIR absorption intensity of TPA-TQT/CH-4T in PBS (pH 8.0, 8.5) solution, I0 is the maximal NIR absorption intensity of TPA-TQT/CH-4T in PBS (pH 7.4) solution, respectively. Data are presented as mean ± s.d. derived from n = 3 independent experiments.