Figure 4.
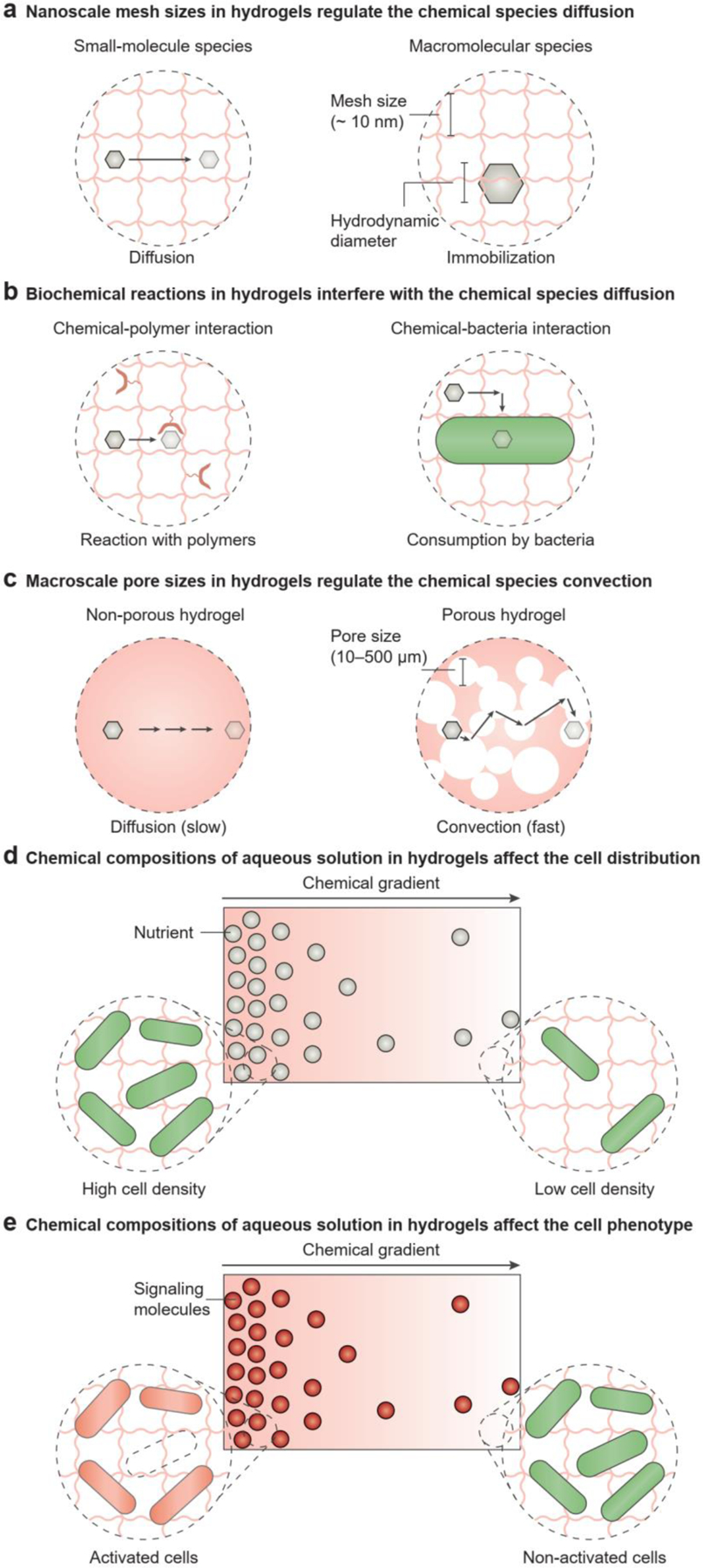
The chemical composition of aqueous solutions in hydrogels affects cell dynamics. a) In non-porous hydrogels the diffusion of chemical species is regulated by the nanoscale mesh of polymer networks. Non-porous hydrogels allow the diffusion of small molecules, but macromolecules are immobilized. b) Biochemical reactions in hydrogels may interfere with the diffusion of chemical species. For example, the chemicals may be consumed if they interact with the polymer network or cells. c) In porous hydrogels, the convection of chemical species is regulated by the macroscale pores. In contrast to the slow diffusion observed in non-porous hydrogels, porous hydrogels allow fast convection of chemicals. d) The chemical composition of aqueous solutions in hydrogels affects cell distribution. For example, the chemical gradient of nutrients sets up the gradient of cell-population densities: A sufficient nutrient supply leads to a high cell density, while an insufficient nutrient supply leads to a low cell density. e) The chemical composition of aqueous solutions in hydrogels affects the cell phenotype. For example, the chemical gradient of signaling molecules causes the population-density gradient of activated cells: A high concentration of signaling molecules leads to cell activation, while a low concentration does not affect the cell phenotypes.
