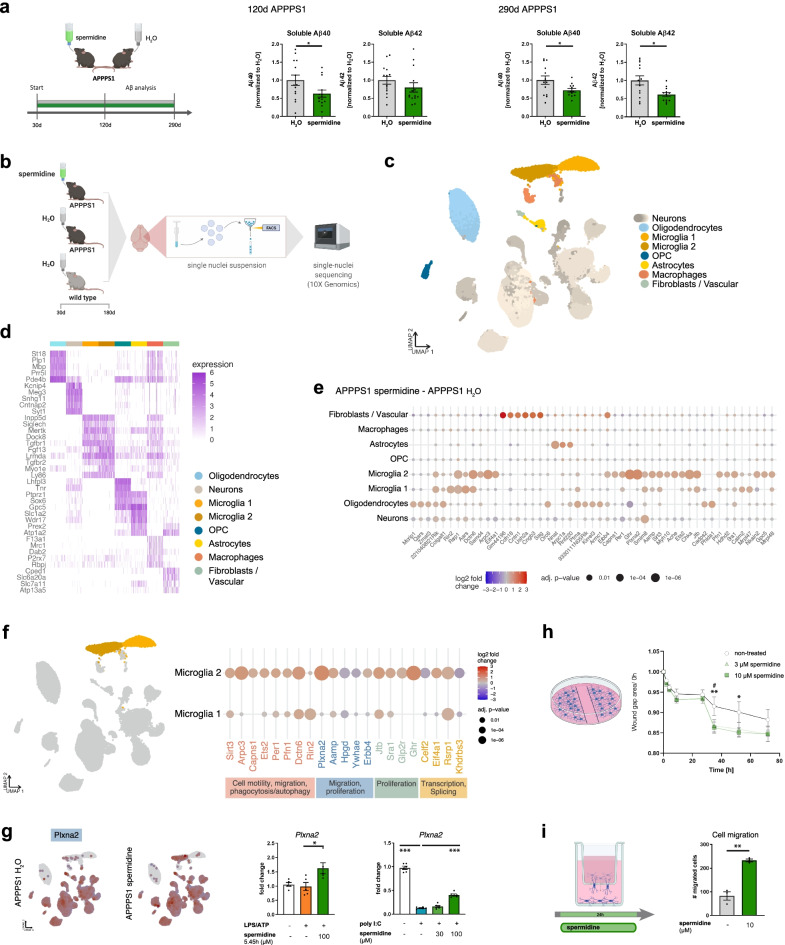
Fig. 1.
Spermidine reduced soluble Aβ and induced transcriptomic alterations in microglia of APPPS1 mice. a APPPS1 mice were treated with 3 mM spermidine via their drinking water starting at 30 days (d) until mice reached an age of 120 days or 290 days according to the depicted treatment scheme. Spermidine-treated APPPS1 mice were compared to non-treated controls (H2O). The Aβ40 and Aβ42 content was measured in the TBS (soluble) fraction of brain homogenates of 120-day-old or 290-day-old spermidine-treated mice and water controls (mixed sex) using electrochemiluminescence (MesoScale Discovery panel). Values were normalized to water controls. 120d APPPS1 H2O (n = 14), 120d APPPS1 spermidine (n = 14), 290d APPPS1 H2O (n = 14), 290d APPPS1 spermidine (n = 12); two‐tailed t‐test, Aβ42 in 120d mice: Mann–Whitney U test. b Single nuclei sequencing of hemispheres harvested from 180-day-old male spermidine-treated APPPS1, H2O APPPS1 and H2O control mice was performed of FACS-sorted DAPI-stained nuclei using the 10x Genomics platform (n = 3). c UMAP embedding and clustering of the snRNA-seq data, together with annotation of the major cell types. d Heatmap showing the top 5 marker genes for 300 cells in each of the major cell types. e Dot plot for the top 50 genes in a cell-type-specific differential expression analysis between spermidine-treated APPPS1 and H2O APPPS1 mice. Color scale indicates log2 fold change, dot size indicates adjusted p value. f Same as e, for selected genes differentially expressed in microglia clusters 1 or 2. Associated pathways are color-coded. g Expression of Plxna2 in APPPS1 spermidine and APPPS1 H2O mice. Color scale indicates normalized expression, grey dots represent no data (left panels). For validation, neonatal microglia were treated with the indicated concentrations of spermidine in combination with LPS (1 µg/ml) and ATP (2 mM) or with poly I:C (50 µg/ml) and the gene expression was assessed by RT-qPCR (right panels). Plxna2 expression was normalized to Actin and displayed as fold change compared to non-treated control cells; n = 5–6, one-way ANOVA, Dunnett’s post hoc test. h Neonatal microglia were pre-treated with 3 or 10 µM spermidine for 15 h. The confluent cell layer was scratched and the scratch area was imaged for 72 h at the indicated timepoints. The gap area normalized to timepoint 0 h is displayed; n = 5–6, two-way ANOVA, Dunnett’s post hoc test. i Neonatal microglia were non-treated or treated with 10 µM spermidine and their migration towards 300 µM ATP was quantified after 24 h using a transwell migration assay; two‐tailed t‐test. *p < 0.05, **p < 0.01, ***p < 0.001