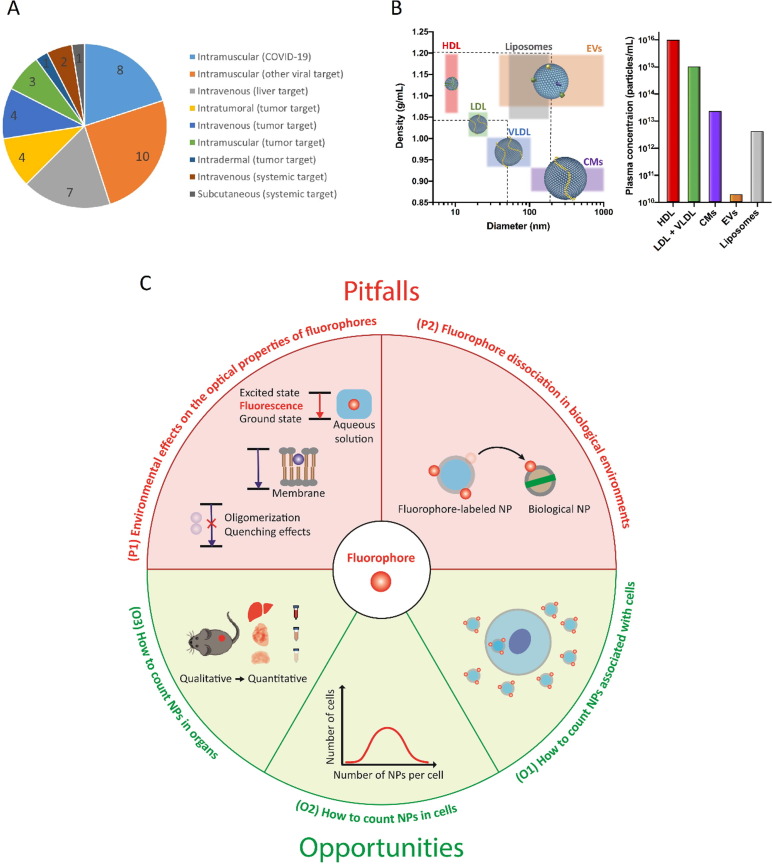
Fig. 7.
A) Administration routes and disease targets used in clinical trials based on LNP delivery of nucleic acid therapies/vaccines. Trials were identified on clinicaltrials.gov using the search string “lipid nanoparticles” and excluding non-LNP-based hits (search date 18.01.2022). Additional trials were identified from Moderna, BioNTech, and CureVac pipelines (search date 18.01.2022), as well as current literature [14], [114]. B) Mapping the physical properties (size and density) of biological (high-density lipoproteins (HDL), low-density lipoproteins (LDL), very low-density lipoproteins, chylomicrons (CM), and extracellular vesicles (EVs)) and liposome-based nanoparticles including their particle concentrations in human plasma (the liposome concentration is based on 1 mM lipid concentration and a size of 100 nm with an average lipid footprint of 0.425 nm2). The LNP density may likely be slightly higher than liposomes due to its nucleic acid cargo [115]. Reprinted with permission from WILEY. J. B. Simonsen, R. Münter, Angew. Chem. Int. Ed. 2020, 59, 12584. [115]. C) Illustration of some of the pitfalls and opportunities in quantitative fluorescence-based nanomedicine studies discussed in [113]. Reprinted from [113] under the terms of Creative Commons license. Copyright 2021 Journal of Controlled Release.