Table 34.
Comprehensive SAR of the reported N, S-heterocyclic scaffolds.
| N, S-Heterocyclic Scaffold | SARs | Key Highlights | References |
|---|---|---|---|
| Thiazoles or Benzothiazoles |  |
|
[183] |
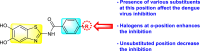 |
|
[184] | |
| Thiazolidinones | 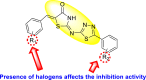 |
|
[190] |
| Benzothiazines | 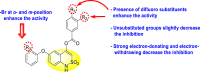 |
|
[195] |
Comprehensive SAR of the reported N, S-heterocyclic scaffolds.
| N, S-Heterocyclic Scaffold | SARs | Key Highlights | References |
|---|---|---|---|
| Thiazoles or Benzothiazoles |  |
|
[183] |
 |
|
[184] | |
| Thiazolidinones |  |
|
[190] |
| Benzothiazines |  |
|
[195] |