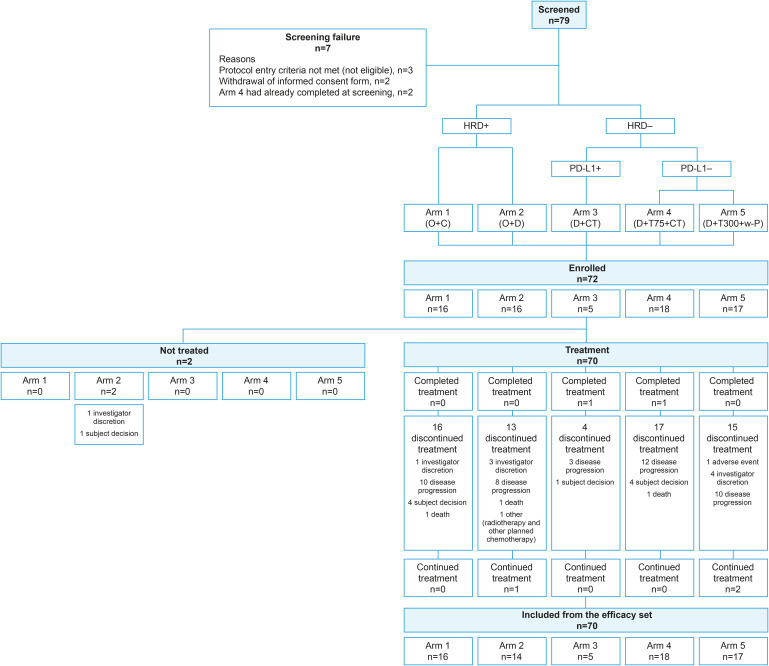
Fig. 1. Study overview and trial profile. Arm 1, O+C (O 200 mg bid + C 30 mg qd); arm 2, O+D (O 300 mg bid + D 1,500 mg q4w); arm 3, D+CT (D 1,500 mg q4w + PLD or topotecan or weekly paclitaxel [6 cycles]) in patients with high PD-L1 expression; arm 4, D+T75+CT (D 1,500 mg q4w + T 75 mg q4w [4 doses] + PLD or topotecan or weekly paclitaxel [4 cycles]) ; arm 5, D+T300+CT (D 1,500 mg q4w + T 300 mg [1 dose] + weekly paclitaxel [60 mg/m2 days 1, 8, and 15 q4w for 4 cycles]).
bid, twice daily; C, cediranib; CT, chemotherapy; D, durvalumab; HRD, homologous recombination deficiency; O, olaparib; PD-L1, programmed death ligand 1; PLD, pegylated liposomal doxorubicin; q4w, every 4 weeks; qd, once daily; T, tremelimumab; T300, tremelimumab 300 mg (1 dose); w-P, weekly paclitaxel.