Abstract
Objectives
Despite the high efficacy of antiretroviral treatment, no drug is free from adverse events (AEs). Efavirenz (EFV) and dolutegravir (DTG) are antiretroviral drugs for which neuropsychiatric adverse events (NPAEs) have been described. This study evaluated the safety and tolerability of DTG-based and EFV-based antiretroviral regimens in HIV-infected patients.
Methods
A retrospective observational study was carried out in HIV-infected patients who started DTG- or EFV-based antiretroviral treatment from January 2008 to December 2018 at a reference hospital in north-western Spain. Epidemiological, clinical and immunovirological data were recorded. A statistical analysis was performed with SPSS software.
Results
A total of 282 DTG- and 148 EFV-based therapies were initiated. During follow-up, statistically significant differences have been found between the rate of patients who discontinued DTG and EFV due to AEs (12.1% vs 35.8%, p<0.001) and the main AEs in both groups, NPAEs (8.2% vs 25.0%, p<0.001). Female gender (OR 2.610 (95% CI 1.327 to 5.133), p=0.005) was associated with discontinuations due to AEs. Patients with documented psychiatric disorders were at higher risk of discontinuation due to NPAEs (OR 4.782 (95% CI 1.190 to 19.220), p=0.027). The multivariate analysis showed a 61.2% risk reduction in benzodiazepine prescriptions in patients treated with DTG. In both groups, patients needed consultation and follow-up in the psychiatry unit (16.9% in the EFV group and 8.9% in the DTG group, p=0.021).
Conclusions
We found a high rate of discontinuations due to AEs and NPAEs, prescription of benzodiazepines and a requirement for consultation in a psychiatric unit in both treatment groups, especially with EFV.
Keywords: HIV & AIDS, clinical pharmacy, infectious diseases, side effects of drugs, adverse effects
Introduction
Epidemiological data regarding neuropsychiatric disorders in patients with HIV are extensive.1 2 Severe mental illness makes individuals more vulnerable to HIV infection, and the proportion of mental and/or substance abuse disorders among people living with HIV is nearly five times greater than in the general population.2 Prevalence estimates of neuropsychiatric disorders among HIV-infected individuals, including substance use disorders, range from 20% to 60%, with variations depending on study designs, and are significantly associated with the presence of lifetime psychiatric disorders prior to the diagnosis of HIV infection.1 2 The main neuropsychiatric disorders observed in patients with HIV are delirium, dementia, schizophrenia, acute stress reactions, anxiety, depression and sleep disorders.1 3 The chance of developing a depressive disorder is two times higher in people living with HIV than in HIV seronegative subjects,4 and there is a marked increase in the rate of suicidal ideation and suicide attempts, three times higher than in the general population.
The introduction of antiretroviral therapy (ART) has revolutionised the treatment of patients with HIV infection. However, many of the antiretroviral drugs used in the treatment of HIV infection have been reported to have neuropsychiatric adverse events (NPAEs).5 These include zidovudine, abacavir, nevirapine, efavirenz (EFV) and the newer class of antiretrovirals, the integrase strand transfer inhibitors.
EFV, a non-nucleoside reverse transcriptase inhibitor of HIV-1, was approved by the American and European drugs regulatory authorities in 1998 and 1999, respectively,6 7 and has been recommended as a preferred third agent for first-line combination ART for 15 years. Nowadays, EFV is considered an alternative regimen due to important CNS adverse effects and the low genetic barrier of non-nucleoside reverse transcriptase inhibitors for the development of drug resistance.8 9 Early reports suggested that EFV may be associated with at least transient NPAEs in more than 50% of treated patients. Reported effects include depression, suicidal ideation, vivid nightmares, anxiety, insomnia, psychosis and cognitive dysfunction.1 5
Integrase strand transfer inhibitors currently available for use are raltegravir (RAL), elvitegravir (EVG), dolutegravir (DTG) and bictegravir. In clinical trials, the rate of adverse events (AEs) such as NPAEs leading to discontinuation was very low (<1%).10–16 However, recently published cohort studies show growing concerns about DTG-induced NPAEs,17–20 with a noted risk observed also for other integrase strand transfer inhibitors (RAL, EVG) but at a lower rate than that observed for DTG.18
EFV is a drug that has been falling into disuse in recent years due to NPAEs while the use of new drugs such as DTG has increased, although concerns about NPAEs due to DTG have recently arisen. In response to the increased risk of neuropsychiatric disorders in the HIV population, we analysed the safety experience with these drugs in our whole patient population. Therefore, this study evaluates and compares the real-life safety of DTG- and EFV-based therapies among a large cohort of naïve and treatment-experienced patients.
Methods
This is an observational study in adult HIV-infected patients who were prescribed DTG or EFV from 1 January 2008 to 31 December 2018 at a reference hospital in northwestern Spain. Only patients who had signed the informed consent and had at least one follow-up visit were included. Patients participating in clinical trials or those transferred from other centres (hospitals or penitentiaries) with the study drugs were excluded from the study. Epidemiological, clinical, immunovirological data and information regarding ART were retrospectively recorded from the electronic patient files. We collected the history of neuropsychiatric disorders according to the clinical electronic history. The time of follow-up was from the day of DTG or EFV initiation until the day of discontinuation or the last visit to the pharmacy service.
Statistical analysis was performed with SPSS v.19. software. Group differences were compared using the Pearson χ2 or Fisher’s exact test, and the Student t-test or the Mann–Whitney U test for categorical and continuous variables, respectively. Repeated measurements were compared using the paired Student t-test or Wilcoxon signed-rank test.
Univariate analyses were performed with all the covariates. Multivariate logistic regression analysis, among the statistically significant univariate covariates plus those found to be clinically relevant, was performed to identify risk factors for discontinuation because of AEs. The time to treatment discontinuation of DTG and EFV due to NPAEs was estimated by the Kaplan–Meier method. P values ≤0.05 were considered statistically significant.
Results
Baseline characteristics of the study population
A total of 282 DTG-based therapies (209 DTG/abacavir/lamivudine, 52 DTG plus tenofovir disoproxil fumarate/emtricitabine and 21 DTG combined with other antiretrovirals) and 148 EFV-based therapies (133 EFV/tenofovir disoproxil fumarate/emtricitabine and 15 EFV plus abacavir/lamivudina) were initiated within the observation period and met the inclusion criteria. The median time of follow-up was 91.8±27.4 months for the EFV group and 34.4±8.4 months for the DTG group.
The majority of patients were Caucasian (91.4%) and men (76.0%), with a median age of 49.3±10.4 years (range 23–83) and treatment-experienced (64.0%). The most common acquisition risk factor for HIV infection was related to sexual activity for both groups. Subjects treated with DTG were more frequently co-infected with hepatitis C virus (HCV) and in the late stage disease of HIV (C stage of Center for Disease Control (CDC) classification). There was a greater percentage of active and ex-drug users in the DTG group, and there was a higher rate of naïve patients in the EFV group (108 vs 47 patients). The epidemiological, clinical, immunological and other baseline characteristics of the two groups are shown in table 1. In treatment-experienced patients, the reasons for switching from previous ART were simplification (44.0%), to avoid intolerance or AEs (34.2%), drug–drug interactions (13.5%) and detectable viral load (5.1%).
Table 1.
Baseline characteristics of the study population
| Variables | EFV (n=148) |
DTG (n=282) |
P value |
| Demographic-epidemiological | |||
| Male (%) | 81.8 | 73.0 | 0.044 |
| Age (mean±SD) (years) | 48.1±9.4 | 49.9±10.8 | |
| 20–40 years (%) | 22.3 | 16.7 | 0.143 |
| 41–60 years (%) | 71.6 | 72.7 | |
| >60 years (%) | 6.1 | 10.6 | |
| Routes of HIV transmission (%) | |||
| MSM | 45.9 | 27.7 | |
| Heterosexual | 35.8 | 29.8 | |
| IDU | 15.5 | 37.9 | |
| Vertical | 0.0 | 1.8 | |
| Unknown | 2.7 | 2.8 | |
| Ethnic origin (%) | |||
| Caucasian | 94.6 | 89.7 | |
| Latin | 4.1 | 8.5 | |
| Black | 1.4 | 1.8 | |
| CDC stage (%) | |||
| A | 80.4 | 61.7 | <0.001 |
| B | 4.7 | 7.4 | |
| C | 14.9 | 30.9 | |
| HIV naïve (%) | 73.0 | 16.7 | <0.001 |
| Co-infections (%) | |||
| Anti-HCV positive | 16.2 | 39.7 | <0.001 |
| HBsAg positive | 6.1 | 2.8 | 0.101 |
| Psychiatric disorders (%) | 20.9 | 24.5 | 0.411 |
| Mixed anxiety-depressive symptoms | 6.1 | 6.0 | |
| Anxiety | 8.1 | 3.2 | |
| Depression | 2.0 | 3.2 | |
| Substance addictive disorders | 0.0 | 3.2 | |
| Drug users (%) | |||
| Never | 86.5 | 65.2 | <0.001 |
| Ex-drug users | 9.5 | 27.3 | |
| Active users | 4.1 | 7.4 | |
| Drug users rehabilitation follow-up (%) | 3.4 | 16.7 | <0.001 |
CDC, Center for Disease Control; DTG, dolutegravir; EFV, efavirenz; IDU, intravenous drug use; MSM, men who have sex with men.
In both groups there was an important percentage of patients with psychiatric disorders (24.5% in the DTG group vs 20.9% of patients in the EFV group, p=0.411) documented before the start of the study. The most frequent psychiatric disorders were mixed anxiety-depressive symptoms (26.0%), anxiety (21.0%), depression (12.0%) and substance addictive disorders (9.0%). Anxiety (8.1%) and mixed anxiety-depressive symptoms (6.1%) were the most common in the EFV group while mixed anxiety-depressive disorders (6.0%), anxiety (3.2%), depression (3.2%) and substance addictive disorders (3.2%) were the most common in the DTG group.
Safety profile in EFV and DTG groups
During follow-up, 83.1% of patients discontinued EFV therapies (43.1% due to AEs, 24.3% to avoid AEs, 12.2% drug–drug interactions, 9.8% lost to follow-up, 6.5% virological failure, 3.3% simplification and 0.8% death (n=1, not related to EFV)). In the case of EFV discontinuations due to AEs (35.8% of the overall group), 69.8% (ie, 25.0% of overall patients treated with EFV) were related to neuropsychiatric disorders, 11.3% to cutaneous toxicity, 7.5% to hypercholesterolaemia, 5.7% to alterations of renal function, 3.8% to gastrointestinal discomfort and 1.9% to haematological toxicity (table 2).
Table 2.
Safety profile in EFV and DTG groups
| EFV (n=148) |
DTG (n=282) |
P value | |
| Discontinuations (%) | 83.1 | 23.8 | <0.001 |
| Due to AEs (%) | 43.1 | 50.8 | |
| NPAEs (%) | 69.8 | 67.7 | |
| Cutaneous toxicity (%) | 11.3 | – | |
| Hypercholesterolaemia (%) | 7.5 | – | |
| Alterations of renal function (%) | 5.7 | 5.9 | |
| Gastrointestinal discomfort | 3.8 | 23.5 | |
| Haematological toxicity | 1.9 | 2.9 | |
| To avoid AEs (%) | 24.3 | 3.0 | |
| Drug–drug interactions (%) | 12.2 | – | |
| Lost to follow-up (%) | 9.8 | 11.9 | |
| Virological failure (%) | 6.5 | 1.5 | |
| Simplification (%) | 3.3 | 13.4 | |
| Death (%) | 0.8 | 17.9 | |
| Pregnant (%) | – | 1.5 |
AEs, adverse events; DTG, dolutegravir; EFV, efavirenz; NPAEs, neuropsychiatric adverse events.
In the DTG group, 23.8% of patients discontinued therapies during follow-up (50.8% due to AEs, 17.9% deaths (n=12, none were considered to be drug-related), 13.4% simplification, 11.9% lost to follow-up, 3.0% to avoid AEs, 1.5% virological failure and 1.5% pregnant). In the case of DTG discontinuations due to AEs (12.1% of the overall group), 67.7% were related to neuropsychiatric disturbances (ie, 8.2% of overall patients treated with DTG), 23.5% to gastrointestinal discomfort, 5.9% to alterations of renal function and 2.9% to haematological toxicity.
Safety profile comparison between groups
Overall, 23.8% of patients discontinued DTG and 83.1% discontinued EFV during the study period (p<0.001). Statistically significant differences were found between the percentage of patients who discontinued DTG and EFV due to AEs (12.1% vs 35.8%, p<0.001) or due to NPAEs (8.2% vs 25.0%, p<0.001). Neuropsychiatric toxicity included insomnia, abnormal dreams, headache, dizziness, nervousness, irascibility, anxiety, depressive symptoms and suicidal ideation in both groups. The time between the start of EFV and DTG and its suspension due to NPAEs was 52.8±48.1 months and 9.5±8.3 months, respectively (p<0.001) (table 3, figure 1).
Table 3.
Safety profile comparison between groups
| EFV (n=148) |
DTG (n=282) |
P value | |
| New drug prescriptions (%) | |||
| Benzodiazepines | 22.3 | 14.5 | 0.059 |
| Antidepressants | 12.8 | 12.1 | 0.937 |
| Antipsychotics | 1.3 | 4.6 | 0.141 |
| Follow-up in the psychiatry unit (%) | 16.9 | 8.9 | 0.021 |
DTG, dolutegravir; EFV, efavirenz.
Figure 1.
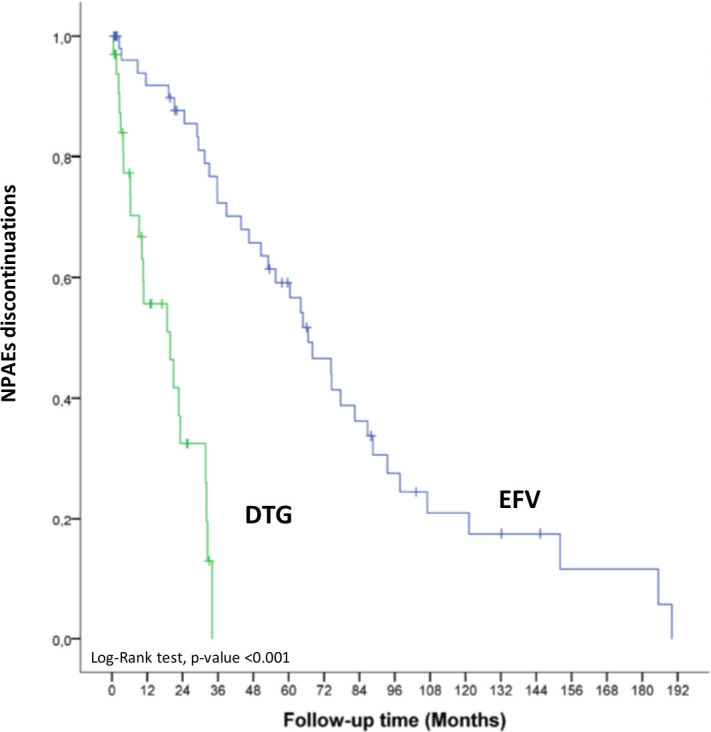
Kaplan–Meier curves showing relationship between discontinuations due to NPAEs after EFV versus DTG initiation. DTG, dolutegravir; EFV, efavirenz; NPAEs, neuropsychiatric adverse events.
In the follow-up period, 17.2% of the patients required a new benzodiazepine prescription after the initiation of DTG or EFV, 12.3% antidepressants and 3.5% antipsychotics. The rate of patients who started benzodiazepines in the EFV group was higher than in the DTG group (22.3% vs 14.5%) but the difference was not statistically significant (p=0.059).
The rate of patients who initiated antidepressants and antipsychotics during the follow-up period was similar in the DTG and EFV groups (12.1% vs 12.8%, p=0.937; 4.6% vs 1.3%, p=0.141). Likewise, no differences were found in the number of patients who discontinued benzodiazepines, antidepressants and antipsychotic treatments after stopping ART with DTG or EFV. There were statistically significant differences in the need for consultation and follow-up in the psychiatry unit after the start of the study drugs (16.9% with EFV vs 8.9% for DTG, p=0.021).
A univariate analysis of risk factors for discontinuations due to AEs and NPAEs in both study groups was performed considering age, gender, race, routes of HIV transmission, treatment experience (naïve vs treatment-experienced), CDC stage, HBV and HCV co-infection, drug use (never, ex-drug user or active user), previous psychiatric disorders and ART (DTG vs EFV). Naïve patients (OR 0.391 (95% CI 0.242 to 0632), p<0.001), those with HCV co-infection (OR 0.497 (95% CI 0.283 to 0.875), p=0.015), those in whom the route of HIV transmission was as an injecting drug user (OR 0.399 (95% CI 0.210 to 0.757), p=0.005) or patients treated with DTG (OR 0.246 (95% CI 0.150 to 0.402), p<0.001) were less likely to interrupt treatment due to AEs. However, HBV co-infection was associated with a higher risk of discontinuation due to AEs (OR 2.914 (95% CI 1.076 to 7.891), p=0.035). Patients over 60 years of age were at a lower risk of treatment discontinuations due to NPAEs (OR 0.036 (95% CI 0.003 to 0.417), p=0.008).
In the multivariate Cox regression model, female gender (OR 2.610 (95% CI 1.327 to 5.133), p=0.005) was significantly associated with discontinuations due to AEs in patients treated with both EFV and DTG. Nevertheless, patients receiving DTG therapy (OR 0.309 (95% CI 0.169 to 0.565), p<0.001) and those in whom the route of HIV transmission was as an injecting drug user (OR 0.435 (95% CI 0.204 to 0.927), p=0.031) were at lower risk of discontinuations due to AEs. Among those treated with EFV, women had a higher risk of discontinuations due to AEs than men (OR 3.311 (95% CI 1.084 to 10.111), p=0.036). However, patients in whom the route of HIV transmission was as an injecting drug user were at lower risk of discontinuation due to AEs (OR 0.190 (95% CI 0.045 to 0.807), p=0.024). No variables were significantly associated with the risk of discontinuation due to AEs in the multivariate analysis of the DTG group.
Patients with documented psychiatric disorders were at higher risk of discontinuation due to NPAEs (OR 4.782 (95% CI 1.190 to 19.220), p=0.027) when the entire study population was analysed. None of the variables analysed were associated with an increased risk of discontinuations due to NPAEs in each of the study groups in the multivariate analyses. The results of the multivariate analyses are shown in table 4.
Table 4.
Multivariate analyses results
| OR (95% CI) | P value | |
| Discontinuations due to AEs | ||
| Female gender | 2.610 (1.327 to 5.133) | 0.005 |
| Discontinuations due to NPAEs | ||
| Previous documented psychiatric disorders | 4.782 (1.190 to 19.220) | 0.027 |
AEs, adverse events; NPAEs, neuropsychiatric adverse events.
A univariate and multivariate analysis, similar to that previously detailed, of risk factors for a new benzodiazepine prescription were performed. In the multivariate analysis, patients treated with DTG were less likely to need a benzodiazepine prescription than those treated with EFV (OR 0.388 (95% CI 0.188 to 0.798), p=0.010), despite the fact that in the DTG group there was a higher rate of drug users who are more likely to be benzodiazepine users.
Discussion
In this cohort of 430 HIV-infected patients who initiated DTG- or EFV-based therapies, we found a high rate of discontinuations, AEs being the main reason in both groups.
The discontinuation rate due to AEs in patients treated with EFV is well documented,21–23 and our results were consistent with the literature published to date (15.6–35.3%). EFV has been the preferred ART for use in naïve patients in international clinical guidelines up to 2015. EFV had earned its preferential position because of its demonstrated efficacy24 and offers the advantage of being the first complete ART marketed in a single tablet regimen combined with tenofovir disoproxil fumarate/emtricitabine. These features, accompanied by affordable cost, may explain the high rate of naïve patients in the EFV group in the observation period. In recent years it has fallen into disuse due to its AEs and the marketing of new drugs with superior efficacy and fewer AEs.10
One of these new antiretrovirals is DTG, which is available in a single tablet regimen and has been positioned as the preferred treatment in the clinical guidelines since 2015 due to its high antiviral potency and a good safety profile shown in clinical trials, together with other integrase inhibitors. However, concerns about the tolerability of DTG have emerged in recent cohorts,17 19 25 which suggests that DTG is associated with a higher incidence of AEs resulting in treatment discontinuation compared with that reported in clinical trials,10–16 especially NPAEs. This unexpected high rate of discontinuations with DTG was seen in our group of patients (12.1%), confirming what we had already seen in a previous study with a smaller sample of patients and with shorter follow-up than in the present study (10.2%).18
In agreement with our previous findings18 and those of other studies,19 25 26 the results show that female gender was associated with a higher risk of treatment interruption for any AE. When we analyse each of the groups, none of the variables has been significantly associated with the risk of discontinuation due to AEs in the DTG group, but in those treated with EFV it is confirmed that female patients were at a higher risk of discontinuations due to AEs (OR 3.311 (95% CI 1.084 to 10.111), p=0.036). This finding is not confirmed by other authors such as Pérez-Molina et al 22 who found an equivalent overall incidence of AEs for both genders in EFV-treated patients.
The present study shows that DTG therapy had a 69.1% lower risk of discontinuations due to AEs than EFV. These data are in line with those reported in a clinical trial comparing DTG and EFV.16 In this trial, the authors reported significantly more discontinuations in the EFV group than in the DTG group, especially because of NPAEs, as found in our study population when we compared both drugs. The NPAEs of EFV are widely described in the literature.1 21 23 However, with DTG treatment there are very different data. While clinical trials and some authors report NPAEs below 1%,10–13 16 27 other authors have found similar rates to those seen in our DTG study group (Hoffman et al 17 reported a rate of discontinuations due to NPAEs of 5.6% and De Boer et al 19 reported a rate of 9.9%) and those that we also described previously (6.9%).18 The main NPAEs reported in DTG clinical trials were insomnia, dizziness, anxiety, abnormal dreams, headache, fatigue, mood changes, nervousness, depression and suicidal ideation.16 28 These kinds of NPAEs were similar to those seen in our study population, although the rates were different, as already commented.
Patients with documented psychiatric disorders had a 4.7 times higher risk of discontinuation due to NPAEs among the entire study population. The baseline psychiatric disorders of patients are not normally collected, so it has not been possible to see the influence of this variable in the treatment discontinuation due to NPAEs. Inconsistent information is found in the literature about the potential impact of previous psychiatric diseases on NPAEs.25 29 Despite the paucity of relevant studies, neuropsychiatric symptoms and syndromes are frequently present among HIV-infected individuals, similar to those in our population before the start of the study drugs. Although psychiatric symptomatology may occur as a consequence of HIV disease, it may also be a risk factor for contracting it.2 The aetiology is likely to be multifactorial, including immune activation due to HIV, alcohol and drug use or antiretroviral toxicities. Studies suggest that the prevalence of neuropsychiatric disorders in patients with HCV is higher than in the general population and that HCV/HIV co-infection may increase cognitive dysfunction.30 In our study population the rate of HCV/HIV co-infected patients was significantly higher in the DTG group. Probably more DTG-based therapy has been prescribed in the co-infected population in order to avoid drug–drug interactions when HCV direct-acting antivirals were prescribed.
The association between the use of illicit drugs and the occurrence of AEs has been described in the literature.26 Conversely, in our study, patients in whom the route of HIV acquisition was as an injecting drug user were at a lower risk of discontinuation, and the actual drug use situation was not identified as an independent risk factor for treatment discontinuation due to AEs.
The reasons for the discordant findings observed in treatment discontinuation because of AEs and NPAEs between some real-life retrospective series, as in our data, and clinical trials are unclear. Numerous factors including the heterogeneity of the study populations, time of follow-up, co-medication and the observational research design in contrast with that of clinical trials designed to provide evidence of efficacy and safety under ideal conditions may partially explain these results. Differences between patients treated with DTG and EFV should be cautiously interpreted as this is a retrospective observational study and therefore the two populations were not comparable. Variables with statistically significant differences between both groups (ie, women, HCV co-infection, rate of active users and ex-drug users) were taken into account in the multivariate analysis.
The main limitations of our study were the small sample size, the single-centre nature of the study and the retrospective observational design, which might have introduced uncontrolled bias. Also, we should take into account the difference in follow-up time in the two groups due to their different marketing dates.
Conclusion
In this study an unexpectedly high rate of discontinuation due to AEs was observed in both the DTG and EFV groups, especially by NPAEs. In particular, female gender and previous documented psychiatric disorders were identified as predictors for discontinuations due to AEs and NPAEs, respectively. A high rate of prescriptions of benzodiazepines and requirement for consultation in a psychiatric unit was seen in both treatment groups, especially in the EFV group. These findings should be considered in the real-life setting when using EFV and DTG, highlighting the AE data for DTG, a drug positioned as preferred in the current clinical guidelines.
What this paper adds.
What is already known on this subject
Prevalence estimates of neuropsychiatric disorders among HIV-infected individuals is higher than in the general population.
Multiple antiretrovirals used in the treatment of HIV infection have been reported to have neuropsychiatric adverse effects,
What this study adds
Efavirenz and dolutegravir have an unexpectedly high rate of discontinuation due to adverse events, especially by neuropsychiatric adverse effects.
Female gender and previous documented psychiatric disorders were identified as predictors for discontinuations due to adverse events and neuropsychiatric adverse effects, respectively
Footnotes
Contributors: NF-B: data collection, analysis and interpretation. Writing of the article: SR-S. Data collection: VB-B. Statistical analysis: LM-F and IM-H. Conception, realisation and development: AC-I, AM-D-C, SL-C, PV-R and EM-R. Data collection: C-SP.
Funding: This work was supported in part by grants from Fundación Profesor Novoa Santos, A Coruña.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The research protocol has been approved by the regional ethic committee (register code 2014/564)
References
- 1. Chaponda M, Aldhouse N, Kroes M, et al. Systematic review of the prevalence of psychiatric illness and sleep disturbance as co-morbidities of HIV infection in the UK. Int J STD AIDS 2018;29:704–13. 10.1177/0956462417750708 [DOI] [PubMed] [Google Scholar]
- 2. Remien RH, Stirratt MJ, Nguyen N, et al. Mental health and HIV/AIDS: the need for an integrated response. AIDS 2019;33:1411–20. 10.1097/QAD.0000000000002227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibarra-Barrueta O. Estudio piloto para desarrollar Y validar un cuestionario basado en El índice de síntomas de VIH. Farm Hosp 2019;03:87–93. [DOI] [PubMed] [Google Scholar]
- 4. Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 2001;158:725–30. 10.1176/appi.ajp.158.5.725 [DOI] [PubMed] [Google Scholar]
- 5. Abers MS, Shandera WX, Kass JS. Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs 2014;28:131–45. 10.1007/s40263-013-0132-4 [DOI] [PubMed] [Google Scholar]
- 6. FDA approves Sustiva (efavirenz) capsules, first once-daily anti-HIV drug. Food and drug administration. Res Initiat Treat Action 1998;4:13–14. [PubMed] [Google Scholar]
- 7. European Medicines Agency . Sustiva, 2018. Available: https://www.ema.europa.eu/en/medicines/human/EPAR/sustiva
- 8. Panel de expertos GeSIDA y Plan Nacional sobre el Sida . Documento de consenso de GeSIDA/Plan nacional sobre el sida respecto al tratamiento antirretroviral en adultos infectados por el virus de la inmunodeficiencia humana (Actualización enero 2019). Available: http://gesida-seimc.org/wp-content/uploads/2019/02/Guia_Tar_Gesida_Ene_2019.pdf
- 9. Panel Members of European AIDS Clinical Society (EACS) . European guidelines for treatment of HIV-positive adults in Europe version 10.0, 2019. Available: https://www.eacsociety.org/files/2019_guidelines-10.0_final.pdf
- 10. Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013;13:927–35. 10.1016/S1473-3099(13)70257-3 [DOI] [PubMed] [Google Scholar]
- 11. Molina J-M, Clotet B, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3B study. Lancet HIV 2015;2:e127–36. 10.1016/S2352-3018(15)00027-2 [DOI] [PubMed] [Google Scholar]
- 12. Castagna A, Maggiolo F, Penco G, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis 2014;210:354–62. 10.1093/infdis/jiu051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013;382:700–8. 10.1016/S0140-6736(13)61221-0 [DOI] [PubMed] [Google Scholar]
- 14. Trottier B, Lake JE, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current art in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, phase IIIB study. Antivir Ther 2017;22:295–305. 10.3851/IMP3166 [DOI] [PubMed] [Google Scholar]
- 15. Orrell C, Hagins DP, Belonosova E, et al. Fixed-dose combination dolutegravir, abacavir, and lamivudine versus ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate and emtricitabine in previously untreated women with HIV-1 infection (ARIA): week 48 results from a randomised, open-label, non-inferiority, phase 3B study. Lancet HIV 2017;4:e536–46. 10.1016/S2352-3018(17)30095-4 [DOI] [PubMed] [Google Scholar]
- 16. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. N Engl J Med Overseas Ed 2013;369:1807–18. 10.1056/NEJMoa1215541 [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann C, Welz T, Sabranski M, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med 2017;18:56–63. 10.1111/hiv.12468 [DOI] [PubMed] [Google Scholar]
- 18. Cid-Silva P, Llibre JM, Fernández-Bargiela N, et al. Clinical experience with the integrase inhibitors dolutegravir and elvitegravir in HIV-infected patients: efficacy, safety and tolerance. Basic Clin Pharmacol Toxicol 2017;121:442–6. 10.1111/bcpt.12828 [DOI] [PubMed] [Google Scholar]
- 19. de Boer MGJ, van den Berk GEL, van Holten N, et al. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS 2016;30:2831–4. 10.1097/QAD.0000000000001279 [DOI] [PubMed] [Google Scholar]
- 20. Borghetti A, Baldin G, Capetti A, et al. Efficacy and tolerability of dolutegravir and two nucleos(t)ide reverse transcriptase inhibitors in HIV-1-positive, virologically suppressed patients. AIDS 2017;31:457–9. 10.1097/QAD.0000000000001357 [DOI] [PubMed] [Google Scholar]
- 21. Leutscher PDC, Stecher C, Storgaard M, et al. Discontinuation of efavirenz therapy in HIV patients due to neuropsychiatric adverse effects. Scand J Infect Dis 2013;45:645–51. 10.3109/00365548.2013.773067 [DOI] [PubMed] [Google Scholar]
- 22. Pérez-Molina JA. Safety and tolerance of efavirenz in different antiretroviral regimens: results from a national multicenter prospective study in 1,033 HIV-infected patients. HIV Clin Trials 2002;3:279–86. 10.1310/3Q91-YT2D-BUT4-8HN6 [DOI] [PubMed] [Google Scholar]
- 23. Fumaz CR, Tuldrà A, Ferrer MJ, et al. Quality of life, emotional status, and adherence of HIV-1-infected patients treated with efavirenz versus protease inhibitor-containing regimens. J Acquir Immune Defic Syndr 2002;29:244–53. 10.1097/00042560-200203010-00004 [DOI] [PubMed] [Google Scholar]
- 24. Amoroso A, Etienne-Mesubi M, Edozien A, et al. Treatment outcomes of recommended first-line antiretroviral regimens in resource-limited clinics. J Acquir Immune Defic Syndr 2012;60:314–20. 10.1097/QAI.0b013e31824e5256 [DOI] [PubMed] [Google Scholar]
- 25. Llibre JM, Montoliu A, Miró JM, et al. Discontinuation of dolutegravir, elvitegravir/cobicistat and raltegravir because of toxicity in a prospective cohort. HIV Med 2019;20:237–47. 10.1111/hiv.12710 [DOI] [PubMed] [Google Scholar]
- 26. Mendes JC, de Fátima Bonolo P, das Graças Braga Ceccato M, et al. Adverse reactions associated with first-line regimens in patient initiating antiretroviral therapy. Eur J Clin Pharmacol 2018;74:1077–88. 10.1007/s00228-018-2472-y [DOI] [PubMed] [Google Scholar]
- 27. Bonfanti P, Madeddu G, Gulminetti R, et al. Discontinuation of treatment and adverse events in an Italian cohort of patients on dolutegravir. AIDS 2017;31:455–7. 10.1097/QAD.0000000000001351 [DOI] [PubMed] [Google Scholar]
- 28. Stellbrink H-J, Reynes J, Lazzarin A, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS 2013;27:1771–8. 10.1097/QAD.0b013e3283612419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fettiplace A, Stainsby C, Winston A, et al. Psychiatric symptoms in patients receiving dolutegravir. J Acquir Immune Defic Syndr 2017;74:423–31. 10.1097/QAI.0000000000001269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perry W, Hilsabeck RC, Hassanein TI. Cognitive dysfunction in chronic hepatitis C: a review. Dig Dis Sci 2008;53:307–21. 10.1007/s10620-007-9896-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.


