Summary
Background
There is no effective treatment for women with unexplained recurrent pregnancy loss (RPL). We aimed to investigate whether treatment with a high dose of intravenous immunoglobulin (IVIG) in early pregnancy can improve pregnancy outcomes in women with unexplained RPL.
Methods
In a double-blind, randomised, placebo-controlled trial, women with primary RPL of unexplained aetiology received 400 mg/kg of IVIG daily or placebo for five consecutive days starting at 4–6 weeks of gestation. They had experienced four or more miscarriages except biochemical pregnancy loss and at least one miscarriage of normal chromosome karyotype. The primary outcome was ongoing pregnancy rate at 22 weeks of gestation, and the live birth rate was the secondary outcome. We analysed all women receiving the study drug (intention-to-treat, ITT) and women except those who miscarried due to fetal chromosome abnormality (modified-ITT). This study is registered with ClinicalTrials.gov number, NCT02184741.
Findings
From June 3, 2014 to Jan 29, 2020, 102 women were randomly assigned to receive IVIG (n = 53) or placebo (n = 49). Three women were excluded; therefore 50 women received IVIG and 49 women received placebo in the ITT population. The ongoing pregnancy rate at 22 weeks of gestation (31/50 [62·0%] vs. 17/49 [34·7%]; odds ratio [OR] 3·07, 95% CI 1·35–6·97; p = 0·009) and the live birth rate (29/50 [58·0%] vs. 17/49 [34·7%]; OR 2·60, 95% CI 1·15–5·86; p = 0·03) in the IVIG group were higher than those in the placebo group in the ITT population. The ongoing pregnancy rate at 22 weeks of gestation (OR 6·27, 95% CI 2·21–17·78; p < 0·001) and the live birth rate (OR 4·85, 95% CI 1·74–13·49; p = 0·003) significantly increased in women who received IVIG at 4–5 weeks of gestation as compared with placebo, but these increases were not evident in women who received IVIG at 6 weeks of gestation. Four newborns in the IVIG group and none in the placebo group had congenital anomalies (p = 0·28).
Interpretation
A high dose of IVIG in very early pregnancy improved pregnancy outcome in women with four or more RPLs of unexplained aetiology.
Funding
The Japan Blood Products Organization.
Keywords: Abortion, Intravenous immunoglobulin, Pregnancy outcome, Recurrent miscarriage, Recurrent pregnancy loss, Unknown aetiology
Research in context.
Evidence before this study
We searched PubMed up until May 23, 2022, using the search terms “unexplained recurrent pregnancy loss”, “therapy”, and “clinical trials” without language restrictions. No standard therapeutic modality for unexplained recurrent pregnancy loss (RPL) has been established. Randomised, double-blind, and placebo-controlled trials (RCTs) found no efficacy of paternal lymphocyte immunisation, prednisone, low dose aspirin and/or heparin, or vaginal progesterone, in women with unexplained RPL. Previous RCTs of intravenous immunoglobulin (IVIG) for unexplained RPL used medium-dose (20–50 g), but the efficacy of the medium-dose IVIG treatment remains unproved.
Added value of this study
This RCT enrolled more severe cases of primary RPL than previous RCTs, who experienced ≥4 miscarriages and at least one miscarriage of a fetus with normal chromosome karyotype. High dose of IVIG (100 g) was administered early in pregnancy starting at 4–6 weeks of gestation. Consequently, this study, for the first time, revealed that high-dose IVIG treatment in women with ≥4 RPLs of unexplained aetiology significantly increased rates of ongoing pregnancy at 22 weeks of gestation and live birth. However, the rates of preterm delivery and fetal growth restriction increased in the IVIG group.
Implications of all the available evidence
High dose of IVIG in very early pregnancy improved pregnancy outcome in women with ≥4 RPLs of unexplained aetiology. This new treatment will give courage and hope to women with severe unexplained RPL who wish to have children. Women who receive high-dose IVIG treatment should be carefully monitored for complications throughout their pregnancy periods. Large scale international clinical trials can be performed to confirm the efficacy of high-dose IVIG treatment on unexplained RPL.
Alt-text: Unlabelled box
Introduction
Recurrent pregnancy loss (RPL) is defined as the loss of ≥2 or ≥3 pregnancies and affects 0·8%–1·4% of couples who attempt to have a baby.1, 2, 3 A variety of factors are involved in the pathogenesis of RPL, such as abnormal uterine morphology, thyroid dysfunction, antiphospholipid syndrome, thrombophilic disorder, and chromosome abnormality. However, the aetiology of >50% of RPL is unknown and is therefore designated as unexplained RPL.4,5 The mechanism underlying the pathology of unexplained RPL remains poorly understood. Recent studies have proposed immunological abnormalities, including natural killer (NK) cells,6, 7, 8 Th1/Th2 balance,8,9 cytokine,9 and regulatory T cells,8,10 for pathophysiology underlying unexplained RPL.
No standard therapeutic modality for unexplained RPL has been established. Randomised, double-blind, and placebo-controlled trials and systematic reviews have found no efficacy of paternal lymphocyte immunization,11 prednisone,12 low dose aspirin and/or heparin,13 or vaginal progesterone,14 in women with unexplained RPL. Some studies have indicated that intravenous immunoglobulin (IVIG) may have therapeutic efficacy for unexplained RPL. Assessment of the efficacy of IVIG treatment in women with ≥2 miscarriages using randomised controlled trials (RCTs) were performed in the 1990s.15, 16, 17, 18, 19, 20 These trials used a medium dose of IVIG, in which 20–50 g of immunoglobulin was infused once, weekly or every 2–4 weeks during follicular phase, early or mid-gestation. Conclusions drawn from these IVIG trials are controversial. Only one study found that IVIG was efficacious;17 however, other studies including RCTs in the 2000s did not.15,16,18, 19, 20, 21, 22 Systematic reviews suggested that medium-dose IVIG treatment was effective in women with secondary RPL,13 but another RCT refuted its efficacy on secondary RPL.24 There are several studies that report beneficial effects of medium-dose IVIG treatment;17,23 however, as most of the studies are not homogeneous in terms of the unexplained RPL definition, gestational age in which start and finish the treatment, and design, they cannot be used together in meta-analysis or systematic review to reach an evidence-based level to be recommended in clinical practice.
It is acknowledged that high-dose IVIG treatment is effective, and this therapy has long been applied to a variety of immune-mediated diseases such as immune thrombocytopenic purpura, Kawasaki's disease, Guillain–Barré syndrome, and myasthenia gravis. The high-dose IVIG treatment (20 g daily for 5 days) in 4–6 weeks of gestation was first reported in 1998,25 and it yielded a high live birth rate of 89·8% among women with a history of ≥4 miscarriages of unexplained aetiology.26 However, these are observational studies, while no RCTs have assessed whether high-dose IVIG treatment improve pregnancy outcome in women with RPL. We assumed that the immunomodulatory effects of high-dose IVIG treatment in early pregnancy restore fecundity in women with unexplained RPL. Therefore, this multicenter, double-blind, randomised, placebo-controlled trial was designed to investigate whether high-dose IVIG treatment in 4–6 weeks of gestation can increase the rates of ongoing pregnancy at 22 weeks of gestation and live birth among women with primary RPL of unexplained aetiology who have a history of ≥4 miscarriages including at least one miscarriage of a fetus with normal chromosome karyotype.
Methods
Study design and participants
The double-blind randomised placebo-controlled trial of IVIG was conducted at 14 study sites including university hospitals and national centres in Japan, wherein the study protocol was approved by the institutional review board of each institution and written informed consent was obtained from all the participants. The pivotal Phase II study was performed in accordance with the principles of the Declaration of Helsinki. The authors assume responsibility for the accuracy and completeness of the data and analyses. The study was conducted in accordance with the Good Clinical Practice guidelines. The monitoring services were outsourced to a contract research organization, MEDISCIENCE PLANNING INC., Tokyo, Japan) independent of the medical institution. Determination of eligibility, reliability of data, and verification of safety were assured through monitoring, including the inspection of source data at least once every month and interviewing investigators and clinical trial collaborators.
Women were eligible to participate if they met all of the following criteria: women who have 1) primary RPL and no children; 2) ≥4 RPLs excluding biochemical pregnancy loss in the count of miscarriages; 3-a) no risk factors of abnormal uterine morphology, thyroid dysfunction with abnormal levels of free T4 or TSH, chromosome abnormality in a couple, a positive test of antiphospholipid antibody (anti‐cardiolipin antibody, anti‐β2-GPI antibody, and lupus anticoagulant), or deficiencies of factor XII, protein S, and protein C, and have experienced at least one miscarriage of a fetus with normal chromosome karyotype; or 3-b) have experienced at least one miscarriage of a fetus with normal chromosome karyotype after having been treated for risk factors as follows: surgical treatment of septate uterus, medical therapy for thyroid dysfunction, and combination therapy with low dose aspirin and heparin for occasional positive of antiphospholipid antibody test, deficiencies of factor XII, protein S, and protein C; and 4) <42 years. Miscarriage was defined as pregnancy loss before 22 weeks of gestation according to Japanese law. At the start of the study in 2014, eligible women were defined as <40 years, and the number of miscarriages with normal chromosome karyotype had to be at least two for women with four or five RPLs and at least one for women with ≥ 6 RPLs. As the number of participants was too small, the protocol was revised in April 2015.
The exclusion criteria were the following: women who have 1) chromosome abnormality in a couple, antiphospholipid syndrome defined according to the updated Sydney classification criteria,27 or the most recent positive test of antiphospholipid antibody; 2) no treatment despite having diabetes mellitus or impaired glucose tolerance; 3) received IVIG for RPL; 4) a history of stillbirth at ≥22 weeks of gestation; 5) treatment for malignancy; 6) thromboembolism; 7) a history of shock or hypersensitivity to immunoglobulin; or 8) IgA deficiency or serum IgA level of <5 mg/dL.
Since no Japanese or South-East Asian has factor V Leiden or prothrombin gene mutation, this study did not assess these coagulation abnormalities for participants.
Randomisation and masking
Participants were randomly assigned in a 1:1 ratio to either the active drug group or the placebo group. The PLAN procedure in SAS was used to generate randomisation codes, and a seed number was randomly specified by the allocation manager. The vials were wrapped with an opaque seal to ensure indistinguishability by pre-assigned physicians or pharmacists who were not involved in drug distribution, administration, or evaluation. Participants, physicians and nurses were blinded. To equalize factors affecting miscarriage, randomisation was done by stratifying the participants on the basis of the number of miscarriages (4 or 5 vs. ≥6) using the minimization method for age (≥ 35 years vs. < 35 years).
Procedures
The active drug used was 5% formulation of intact type human immunoglobulin G (Kenketsu Venoglobulin IH®, the Japan Blood Products Organization). Physiological saline was used as placebo. The active drug of 400 mg/kg or placebo of 8 mL/kg was administered by intravenous drip infusion for five consecutive days. Treatment was initiated at 4 to 6 weeks and 6 days of gestation after gestational sac was identified by ultrasonography. If miscarriages occurred, chromosome karyotype of the villi was performed wherever possible using G-banding or microarray methods.
Outcomes
Two populations were analyzed: all women who received the study drug (intention-to-treat, ITT) and women who received the study drug excluding those who miscarried due to fetal chromosome abnormality (modified-ITT). The primary outcome was the ongoing pregnancy rate at 22 weeks of gestation. The live birth rate was defined as secondary outcomes.
Statistical analysis
The sample size was calculated based on the results of an epidemiological survey of RPL in Japan. The live birth rates in women with four, five, and six prior miscarriages were 65·0%, 58·8%, and 34·2%, respectively.5 The live birth rate was 89.8% when 100 g of immunoglobulin was administered in women with ≥4 miscarriages.26 The sample size was calculated assuming live birth rates of 42%–48% (placebo) and 75% (IVIG) with α = 0·05 and β = 0·20. A study with 40 women per group has 80% power. As miscarriages with fetal chromosome abnormality are determined ex post facto, enrollment was continued until the final number of participants increased by approximately 20%. The ongoing pregnancy rate at 22 weeks of gestation, live birth rate, and their 95% confidence interval [CI] were calculated. Fisher's exact test was used to compare the two groups. To estimate ongoing pregnancy rates, Kaplan-Meier curves were plotted for the pregnancy period. Miscarriage and stillbirth were defined as events, and those who had a live birth were censored regardless of preterm or full-term delivery. The ongoing pregnancy rates at 12, 22, 28, and 34 weeks of gestation were estimated, and the IVIG-to-placebo hazard ratio was calculated. This study is registered with ClinicalTrials.gov number, NCT02184741.
Role of the funding source
This study was funded by the Japan Blood Products Organization. The study funder contributed in study design and data interpretation, but had no role in data collection, analysis, writing of the manuscript, or the decision to submit. The study funder remained blinded to individual treatment allocation throughout the study. All authors had full access to all the data in the study and accepted responsibility to submit for publication.
Results
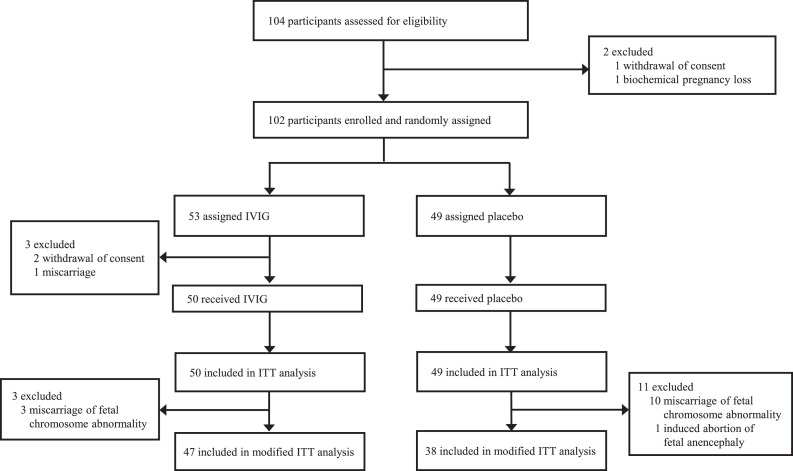
From June 3, 2014 to Jan 29, 2020, 104 women were assessed for eligibility, and 102 women were randomly assigned to receive IVIG (n = 53) or placebo (n = 49); three women in the IVIG group withdrew consent or had an early miscarriage before infusion. Of the remaining 99 women, 50 women received IVIG and 49 women received placebo in the ITT population (Figure 1). These study drugs were started from 4 weeks and 3 days to 6 weeks and 6 days of gestation. The baseline characteristics and protocol adherence were similar in the two groups (Table 1).
Figure 1.
Trial profile. IVIG, intravenous immunoglobulin; ITT, intention-to-treat.
Table 1.
Baseline characteristics of study participants.
| Participant characteristics | IVIG n=50 (%) |
Placebo n=49 (%) |
|---|---|---|
| Age, years | 35·2 ± 3·7 | 35.0 ± 4.0 |
| < 35 | 21 (42·0) | 22 (44·9) |
| ≥ 35 | 29 (58·0) | 27 (55·1) |
| Body weight, kg | 56·3 ± 9·0 | 56·4 ± 11·0 |
| Number of prior miscarrige (range) | 5·1 ± 1·6 (4-11) | 5·2 ± 1·7 (4-11) |
| 4 or 5 times | 36 (72·0) | 34 (69·4) |
| 6 times or more | 14 (28·0) | 15 (30·6) |
| Latest weeks of gestation in past miscarriges | ||
| < 12 weeks | 43 (86·0) | 43 (87·8) |
| ≥ 12 weeks and < 22 weeks | 7 (14·0) | 6 (12·2) |
| Weeks of gestation when gestational sac was identified | 5 weeks, 0 day ± 3 days |
5 weeks, 1 day ± 3 days |
| Weeks of gestation at the start of drug treatment | 5 weeks, 5 days ± 4 days |
5 weeks, 4 days ± 3 days |
| 4 weeks | 3 (6·0) | 3 (6·1) |
| 5 weeks | 31 (62·0) | 33 (67·3) |
| 6 weeks | 16 (32·0) | 13 (26·5) |
Plus–minus values are means ± SD. IVIG, intraveous immunoglobulin.
After treatment, 19 women in the IVIG group and 31 in the placebo group had miscarriages. Of the 19 miscarriages in the IVIG group, 12 had normal chromosome karyotype, three had numerical chromosome abnormality, two were unknown due to inadequate specimen quality, and two were not analysed due to spontaneous evacuation of the abortus. Of the 31 miscarriages in the placebo group, 20 had normal chromosome karyotype, ten had numerical chromosome abnormality, and one was unknown.
One pregnancy with fetal anencephaly in the placebo group was terminated by induced abortion. The remaining 47 women in the IVIG group and 38 in the placebo group were included in the modified-ITT population (Figure 1).
The ongoing pregnancy rate at 22 weeks of gestation (31/50, 62·0%) in the IVIG group was higher than that (17/49, 34·7%) in the placebo group in the ITT population (odds ratio [OR] 3·07, 95% CI 1·35–6·97; p = 0.009). The live birth rate (29/50, 58·0%) in the IVIG group were higher than that (17/49, 34·7%) in the placebo group (OR 2·60, 95% CI 1·15–5·86; p = 0·03). The rates of ongoing pregnancy and live birth were not statistically different between IVIG (47 women) and placebo (38 women) groups in the modified-ITT population (Table 2).
Table 2.
Pregnancy outcomes.
| Intention-to-treat population |
Fisher's exact test |
||||
|---|---|---|---|---|---|
| Administration | Ongoing pregnancy at 22 weeks of gestation– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Ongoing pregnancy at 22 weeks of gestation | Placebo | 17/49 (34·7) | 21·7–49·6 | 0·009 | 3·07 (1·35-6·97) |
| IVIG | 31/50 (62·0) | 47·2–75·3 | |||
| Administration | Live births– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Live birth | Placebo | 17/49 (34·7) | 21·7–49·6 | 0·03 | 2·60 (1·15-5·86) |
| IVIG | 29/50 (58·0) | 43·2–71·8 | |||
|
Modified intention-to-treat population |
Fisher's exact test |
||||
| Administration | Ongoing pregnancy at 22 weeks of gestation– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Ongoing pregnancy at 22 weeks of gestation | Placebo | 17/38 (44·7) | 28·6–61·7 | 0·08 | 2·39 (0·99-5·77) |
| IVIG | 31/47 (66·0) | 50·7–79·1 | |||
| Administration | Live births– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Live birth | Placebo | 17/38 (44·7) | 28·6–61·7 | 0·13 | 1·99 (0·83-4·47) |
| IVIG | 29/47 (61·7) | 46·4–75·5 | |||
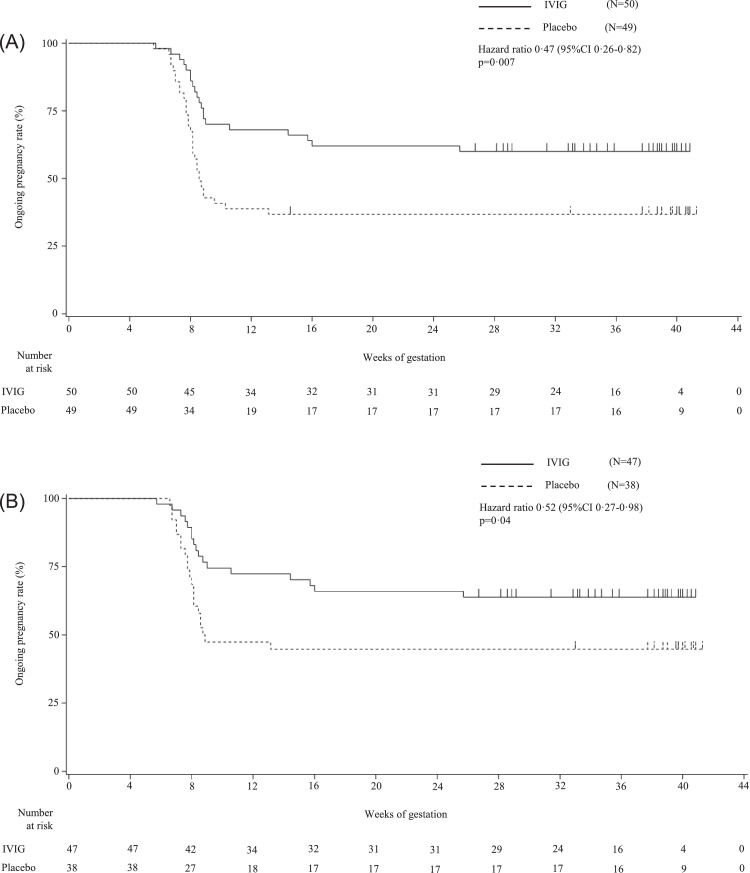
The IVIG-to-placebo hazard ratios for the ongoing pregnancy were 0·47 (95% CI 0·26–0·82; p = 0·007) in the ITT population and 0·52 (95% CI 0·27–0·98; p = 0·04) in the modified-ITT population, respectively (Figure 2).
Figure 2.
Kaplan-Meier curves of ongoing pregnancy rates. Kaplan-Meier curves of ongoing pregnancy rates for each of the IVIG and placebo groups in the intention-to-treat population (Panel A) and in the modified intention-to-treat population (Panel B). Miscarriage and stillbirth were defined as events, and pregnant women who had a live birth or an induced abortion due to fetal anomaly were censored and depicted as marks on the curve. IVIG, intravenous immunoglobulin.
Panel A: In the intention-to-treat population, Kaplan-Meier estimates of the ongoing pregnancy rates at 12, 22, 28, and 34 weeks of gestation were 38·8%, 36·7%, 36·7%, and 36. ·7% in the placebo group; and 68·0%, 62·0%, 60·0%, and 60·0% in the IVIG group, respectively. The IVIG-to-placebo hazard ratio for the ongoing pregnancy rate was 0·47 (95% CI: 0·26–0·82), and the log-rank test indicates a significant difference (p = 0·007).
Panel B: In the modified intention-to-treat population, Kaplan-Meier estimates of the ongoing pregnancy rates at 12, 22, 28, and 34 weeks of gestation were 47·4%, 44·7%, 44·7%, and 44·7% in the placebo group; and 72·3%, 66·0%, 63·8%, and 63·8% in the IVIG group, respectively. The IVIG-to-placebo hazard ratio for the ongoing pregnancy rate was 0·52 (95% CI: 0·27–0·98), and the log-rank test indicates a significant difference (p = 0·04).
Among women who had live births, gestational age at delivery was earlier in the IVIG group than the placebo group, and the rates of preterm delivery (13/29 [44·8%] vs. 1/17 [5·9%]) and fetal growth restriction (10/29 [34·5%] vs. 0/17 [0%]) were higher in the IVIG group compared with the placebo group. The number of live births was 30 including one pair of twins in the IVIG group and 17 in the placebo group. In the IVIG group except twins, birth weight was lower, and the rate of small for gestational age (12/28 [35·7%] vs. 0/17 [0%]) was higher compared with the placebo group. The karyotypes of three miscarriages in the IVIG group and ten in the placebo group had numerical chromosome abnormalities, whereas those of four miscarriages in the IVIG group and one in the placebo group were unknown. The rates of normal/abnormal chromosome karyotypes of miscarriages or congenital anomalies were not different between the two groups. Four newborns in the IVIG group and none in the placebo group had congenital anomalies (p = 0·28). Normal chromosome karyotypes of a total of 32 miscarriages consisted of 46,XX (n = 23) and 46,XY (n = 9). The disproportion might be derived from contamination of maternal tissues to some extent. (Table 3). Although preeclampsia was observed in four (8·0%) of 50 women in the IVIG group and one (2·0%) of 49 women in the placebo group (p = 0·36), there were no thromboembolic events. Twenty-three (46·0%) of 50 women receiving IVIG had mild adverse events including elevated liver enzymes in nine (18·0%), headache in four (8·0%), skin rash in four (8·0%), and fever in two (4·0%) women, while a total of three (6·1%) of 49 women in the placebo group had adverse events.
Table 3.
Comparison of pregancy outcomes in intention-to-treat population.
| Pregnancy outcomes | IVIG | Placebo | p value |
|---|---|---|---|
| n=50 (%) | n=49 (%) | ||
| Ongoing pregnancy at 22 weeks of gestation | 31 (62·0) | 17(34·7) | 0·009* |
| Live birth | 29 (58·0) | 17 (34·7) | 0·03* |
| Gestational age at delivery | 36 weeks, 1 day ± 4 weeks, 0 days | 39 weeks, 2 days ± 2 weeks, 0 days | 0·004⁎⁎ |
| Mode: Vaginal delivery | 12 (41·4) | 7 (41·2) | 1·00* |
| Cesarean section | 17 (58·6) | 10 (58·8) | 1·00* |
| Preterm delivery (< 37 weeks) | 13 (44·8) | 1 (5·9) | 0·007* |
| Fetal growth restriction | 10 (34·5) | 0 (0·0) | 0·008* |
| Miscarriage | 19 (38·0) | 31 (63·3) | 0·02* |
| Gestational age at miscarriage | 9 weeks, 2 days ± 2 weeks, 6 days | 8 weeks, 0 days ± 1 week, 2 days | 0·045⁎⁎ |
| Time of miscarriage | |||
| < 12 weeks | 16 (84·2) | 30 (96·8) | 0·15* |
| ≥ 12 weeks and < 22 weeks | 3 (15·8) | 1 (3·2) | 0·28* |
| Chromosome karyotype of miscarriage | |||
| Normal | 12 (63·2) | 20 (64·5) | 0·49* |
| Abnormal | 3 (15·8) | 10 (32·3) | |
| Unknown1) | 2 (10·5) | 1 (3·2) | - |
| Not tested2) | 2 (10·5) | 0 (0·0) | - |
| Stillbirth | 13) (2·0) | 0 (0·0) | 0·39* |
| Unknown outcome due to discontinuation | 14) (2·0) | 15) (2·0) | 1·00* |
| Newborns | IVIG | Placebo | p-value |
| n=286) (%) | n=17 (%) | ||
| Birth weight, g | 2246·4 ± 962·5 | 3071·6 ± 463·4 | 0·002⁎⁎ |
| Apgar score at 5 minutes | 8·7 ± 1·1 | 9·1 ± 0·6 | 0·13⁎⁎ |
| Small for gestational age | 12 (35·7) | 0 (0·0) | 0·001* |
| Congenital anomaly | 47) (14·3) | 0 (0·0) | 0·28* |
Plus–minus values are means ± SD.
Fisher's exact test.
Student's t-test.
1) Due to inadequate specimen quality
2) Due to spontaneous evacuation of the abortus
3) A stillbirth occurred at 25 weeks and 5 days of gestation due to abnormal umbilical cord coiling. The chromosome karyotype was normal.
4) A case discontinued the study at 27 weeks and 0 days of gestation due to adverse events of threatened preterm labor. The follow-up survey of adverse events confirmed live birth at 34 weeks and 1 day of gestation.
5) A case discontinued the study at 14 weeks and 4 days of gestation due to adverse events of fetal anencephaly, and the pregnancy was terminated by induced abortion. The chromosome karyotype was normal.
6) One case of twin pregnancy is excluded.
7) One case each of atrial septal defect, cleft lip and palate, congenital hearing loss, ventricular septal defect/cerebral cyst
To assess the relationship between the timing of treatment initiation and pregnancy outcomes, the subjects were divided into women who started treatment at 4 or 5 weeks of gestation and women who started at 6 weeks. In the ITT population, the rates of ongoing pregnancy at 22 weeks of gestation (OR 6·27, 95% CI 2·21–17·78; p < 0·001) and live birth (OR 4·85, 95% CI 1·74–13·49); p = 0·003); and the rates of ongoing pregnancy at 22 weeks of gestation (OR 5·40, 95% CI 1·79–16·30; p = 0·004) and live birth (OR 4·03, 95% CI 1·37–11·84; p = 0·02) in the modified-ITT population, were significantly higher in the IVIG group than the placebo group in women who started at 4 or 5 weeks of gestation, but not in women who started at 6 weeks (Table 4).
Table 4.
Time of treatment initiation and pregnancy outcome.
| Intention-to-treat population |
Fisher's exact test |
|||||
|---|---|---|---|---|---|---|
| Time at the start of administration | Administration | Ongoing pregnancy at 22 weeks of gestation– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Ongoing pregnancy at 22 weeks of gestation | 4 or 5 weeks | Placebo | 9/36 (25·0) | 12·1–42·2 | <0·001 | 6·27 (2·21-17·78) |
| IVIG | 23/34 (67·6) | 49·5–82·6 | ||||
| 6 weeks | Placebo | 8/13 (61·5) | 31·6–86·1 | 0·71 | 0·63 (0·14-2·76) | |
| IVIG | 8/16 (50·0) | 24·7–75·3 | ||||
| Time at the start of administration | Administration | Live births– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Live birth | 4 or 5 weeks | Placebo | 9/36 (25·0) | 12·1–42·2 | 0·003 | 4·85 (1·74-13·49) |
| IVIG | 21/34 (61·8) | 43·6–77·8 | ||||
| 6 weeks | Placebo | 8/13 (61·5) | 31·6–86·1 | 0·71 | 0·66 (0·141-2·76) | |
| IVIG | 8/16 (50·0) | 24·7–75·3 | ||||
|
Modified intention-to-treat population |
Fisher's exact test |
|||||
| Time at the start of administration | Administration | Ongoing pregnancy at 22 weeks of gestation– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Ongoing pregnancy at 22 weeks of gestation | 4 or 5 weeks | Placebo | 9/28 (32·1) | 15·9–52·4 | 0·004 | 5·40 (1·79-16·30) |
| IVIG | 23/32 (71·9) | 53·3–86·3 | ||||
| 6 weeks | Placebo | 8/10 (80·0) | 44·4–97·5 | 0·23 | 0·29 (0·05-1·82) | |
| IVIG | 8/15 (53·3) | 26·6–78·7 | ||||
| Time at the start of administration | Administration | Live births– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Live birth | 4 or 5 weeks | Placebo | 9/28 (32·1) | 15·9–52·4 | 0·02 | 4·03 (1·37-11·84) |
| IVIG | 21/32 (65·6) | 46·8–81·4 | ||||
| 6 weeks | Placebo | 8/10 (80·0) | 44·4–97·5 | 0·23 | 0·26 (0·04-1·82) | |
| IVIG | 8/15 (53·3) | 26·6–78·7 | ||||
The subjects were also divided into women with four or five prior miscarriages and women with ≥6 prior miscarriages to assess the relationship between the number of miscarriages and pregnancy outcomes. In the ITT population, the rates of ongoing pregnancy at 22 weeks of gestation (OR 10·00, 95% CI 1·80–55·36; p = 0·009) and live birth (OR 7·20, 95% CI 1·35–38·32; p = 0·03); and the rates of ongoing pregnancy at 22 weeks of gestation (OR 8·33, 95% CI 1·47–47·23; p = 0·04) and live birth (OR 6·00, 95% CI 1·11–32·55; p = 0·05) in the modified-ITT population, were significantly higher in the IVIG group than the placebo group in women with ≥6 miscarriages (Table 5).
Table 5.
Number of previous miscarriages and pregnancy outcome.
| Intention-to-treat population |
Fisher's exact test |
|||||
|---|---|---|---|---|---|---|
| Number of previous miscarriages | Administration | Ongoing pregnancy at 22 weeks of gestation– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Ongoing pregnancy at 22 weeks of gestation | 4 or 5 times | Placebo | 14/34 (41·2) | 24·6–59·3 | 0·23 | 2·00 (0·77-5·18) |
| IVIG | 21/36 (58·3) | 40·8–74·5 | ||||
| 6 times or more | Placebo | 3/15 (20·0) | 4·3–48·1 | 0·009 | 10·00 (1·80-55·63) | |
| IVIG | 10/14 (71·4) | 41·9–91·6 | ||||
| Number of previous miscarriages | Administration | Live births– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Live birth | 4 or 5 times | Placebo | 14/34 (41·2) | 24·6–59·3 | 0·24 | 1·79 (0·69-4·61) |
| IVIG | 20/36 (55·6) | 38·1–72·1 | ||||
| 6 times or more | Placebo | 3/15 (20·0) | 4·3–48·1 | 0·03 | 7·20 (1·35-38·32) | |
| IVIG | 9/14 (64·3) | 35·1–87·2 | ||||
|
Modified intention-to-treat population |
Fisher's exact test |
|||||
| Number of previous miscarriages | Administration | Ongoing pregnancy at 22 weeks of gestation– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Ongoing pregnancy at 22 weeks of gestation | 4 or 5 times | Placebo | 14/25 (56·0) | 34·9–75·6 | 0·6 | 1·38 (0·48-3·98) |
| IVIG | 21/33 (63·6) | 45·1–79·6 | ||||
| 6 times or more | Placebo | 3/13 (23·1) | 5·0–53·8 | 0·02 | 8·33 (1·47-47·23) | |
| IVIG | 10/14 (71·4) | 41·9–91·6 | ||||
| Number of previous miscarriages | Administration | Live births– no./total no. (%) | 95% CI | p-value | Odds ratio (95% CI) | |
| Live birth | 4 or 5 times | Placebo | 14/25 (56·0) | 34·9–75·6 | 0·79 | 1·21 (0·42-3·47) |
| IVIG | 20/33 (60·6) | 42·1–77·1 | ||||
| 6 times or more | Placebo | 3/13 (23·1) | 5·0–53·8 | 0·05 | 6·00 (1·11-32·55) | |
| IVIG | 9/14 (64·3) | 35·1–87·2 | ||||
This study included participants who had experienced at least one miscarriage of a fetus with normal chromosome karyotype after having been treated for risk factors as follows: surgical treatment of septate uterus (IVIG 1/50 vs. placebo 1/49), medical therapy for thyroid dysfunction (IVIG 3/50 vs. placebo 4/49), and combination therapy with low dose aspirin and heparin for occasional positive of antiphospholipid antibody test (IVIG 0/50 vs. placebo 1/49), deficiencies of factor XII (IVIG 3/50 vs placebo 0/49), protein S (IVIG 5/50 vs. placebo 3/49), and protein C (none). The other participants had no risk factors (IVIG 38/50 vs. placebo 40/49).
Discussion
Previous RCTs for RPL of unexplained aetiology used medium-dose IVIG treatment (20–50 g, once, weekly or every 2–4 weeks) during follicular phase, early or mid-gestation in women with ≥2–≥3,15, 16, 17, 18, 19, 20,22 or ≥4 prior miscarriages.21,24 However, the efficacy of these medium-dose IVIG treatments remains unproved.15, 16, 17, 18, 19, 20, 21, 22, 23, 24 The present study enrolled more severe cases of primary RPL than previous RCTs, who experienced ≥4 miscarriages and at least one miscarriage of a fetus with normal chromosome karyotype. To make RPL participants more homogeneous, only primary RPL was enrolled. In addition, for the first time, high dose of IVIG (20 g daily for 5 days) was administered early in pregnancy starting at 4–6 weeks of gestation. Consequently, this RCT revealed that high-dose IVIG treatment in women with ≥4 RPLs of unexplained aetiology significantly increased rates of ongoing pregnancy at 22 weeks of gestation and live birth in the ITT population.
The efficacy of IVIG treatment on the rates of ongoing pregnancy at 22 weeks of gestation and live birth was found in severe cases with ≥6 RPLs in the ITT and modified-ITT populations. These results suggest that severe cases of RPL may have undetermined aetiologies to a greater extent for which IVIG treatment could be effective. The time of treatment initiation was also associated with the efficacy of IVIG treatment. The efficacy on the rates of ongoing pregnancy at 22 weeks of gestation and live birth was more evident in women with administration started at 4 or 5 weeks of gestation, but not in women with administration started at 6 weeks of gestation in the ITT and modified-ITT populations. This RCT first demonstrated that high-dose IVIG treatment started at 4–5 weeks of gestation is especially effective on women with ≥4 RPLs of unexplained aetiology. A recent meta-analysis of RCTs found that IVIG treatment increased live birth rates when initiated prior to conception.28
In the present study, high-dose IVIG treatment was well tolerated in most women, and none of them discontinued the treatment due to adverse effects. However, the gestational age at delivery was earlier, and the rates of preterm delivery and fetal growth restriction were higher in the IVIG group compared with the placebo group. Similarly, the birth weight was lower, and the rate of small for gestational age was higher in the IVIG group compared with the placebo group. High-dose IVIG treatment in very early pregnancy may be insufficient to yield full-term birth or normal fetal growth among severe cases with unexplained RPL in whom the treatment could be effective in preventing early miscarriages. There could be two main reasons for increased rates of preterm delivery and fetal growth restriction in the IVIG group: 1) either the substantial doses of IVIG in very early pregnancy could negatively affect trophoblast invasion in the uterus, which will only be unveiled in the third trimester, or 2) alternatively, high-dose IVIG treatment may rescue some fetuses that would otherwise have been miscarried due to immune disturbances, but the treatment only suppresses the immune disturbances partially increasing the risk of later immune injury to the placenta and fetal growth restriction. A previous study also found the high rates of preterm delivery and fetal growth restriction in women with unexplained RPL who received high-dose IVIG.26 To clarify the reason for high rates of these adverse pregnancy outcomes in unexplained RPL women with high-dose IVIG treatment, further investigations are necessary.
High-dose IVIG treatment has long been applied to a variety of immune-mediated diseases. Many distinct but non-mutually exclusive mechanisms of action, including antiinflammation, suppression of autoantibodies and complements, blockade of FcRn and FcγRn, up-regulation of inhibitory FcγRIIB, modulation of monocytes, macrophages, dendritic cells, natural NK cells, T cells, B cells, and endothelial cells, account for the immunomodulatory effects of IVIG treatment.29,30 Aberrant immunities of NK cells, Th1/Th2 balance, cytokine, and regulatory T cells at the fetomaternal interface and/or in the maternal blood were proposed for aetiologies of unexplained RPL.6, 7, 8, 9, 10 In a mouse model of miscarriage induced by polyinosinic–polycytidylic acid, a high dose of intact type- immunoglobulin but not a medium dose or Fab- immunoglobulin restored fecundity through macrophages together with a reduction of TNF-α and IFN-γ expressions in the placenta.31 Similarly, a high dose of intact type- immunoglobulin in an early period reduced miscarriages through NK cells in a mouse model of miscarriage induced by lipopolysaccharide.32 High-dose IVIG treatment starting at 4–5 weeks of gestation might effectively restore normal immune environment, while the treatment starting at 6 weeks of gestation would be too late to yield the efficacy to restore fecundity. Modification of immune function by a high dose of IVIG during early pregnancy might reduce the number of miscarriages in humans, but the mechanism is still unknown and further investigation is required.
In the modified-ITT population, the efficacy of IVIG treatment on the rates of ongoing pregnancy at 22 weeks of gestation or live birth did not reach statistical significance, since a total of 13 pregnancies were excluded due to chromosome abnormality of miscarriages. The ratio of miscarriage with abnormal chromosome karyotype in the IVIG group (3/50, 6·0%) was lower than that in the placebo group (10/49, 20·4%, p = 0·03). This is potentially a part of IVIG treatment effect, and the IVIG might have a preventative role in averting miscarriage with abnormal chromosome karyotype increasing the ratio of live birth, since transferred mosaic embryos with abnormal chromosome karyotype can develop into healthy euploid newborns in IVF.33 Further investigation is required.
In conclusion, high dose of IVIG in very early pregnancy improved pregnancy outcome in women with ≥4 RPLs of unexplained aetiology. This new treatment will give courage and hope to women with severe unexplained RPL who wish to bear children. However, this trial has several limitations and potential bias. To exclude women who occasionally experienced repeated miscarriages of abnormal chromosome karyotype as much as possible, this trial enrolled severe cases of primary RPL who experienced ≥4 miscarriages of unexplained aetiology and at least one miscarriage with normal chromosome karyotype. These inclusion criteria were most severe compared with previous trials of IVIG. Therefore, it took six years and three months to follow up pregnancies of 99 participants; however, this number was not large. The efficacy of high-dose IVIG treatment was not assessed for secondary RPL. Women who receive high-dose IVIG treatment in very early pregnancy should be carefully monitored for complications throughout their pregnancy periods. Large scale international clinical trials can be performed to confirm the efficacy of high-dose IVIG treatment in very early pregnancy on severe cases of unexplained RPL.
Contributors
H.Y., M.D., S.S., T.T., and H.Y. conceived and designed the study. M.D., S.S., T.T., M.M., T.S., T.N., K.T., M.N., S.Y., K.E., M.T., K.M., R.H., A.F., K.T., K.S., and TE acquired the data. H.Y., S.S., T.T., and H.Y. analysed and interpreted the data. H.Y., M.D., S.S., T.T., and H.Y. verified the underlying data. All authors had full access to all the data in the study and accepted responsibility to submit for publication.
Data sharing statement
Data from this study will be available after approval of manufacturing and marketing from the Japanese regulatory authorities or three years after the completion of this study, whichever is later. Documentation including the protocol, statistical analysis plan, and informed consent document will be made available. Data will be made available to investigators after the proposal for use of the data has been approved. Proposals for the use of data should be addressed to the following yata-hiroaki@jbpo.or.jp.
Declaration of interests
We declare no competing interests.
Acknowledgments
Study drugs were packaged and provided by the Japan Blood Products Organization. We thank all participants for their contributions to this study and thank Tomoyuki Fujii, M.D., Kiyoko Kato, M.D., Hidetaka Katabuchi, M.D., for study design, study management, and oversight. The study was funded by The Japan Blood Products Organization.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101527.
Appendix. Supplementary materials
References
- 1.The Royal College of Obstetricians and Gynaecologists; London: 2011. Royal College of Obstetricians and Gynecologists: The Investigation and Treatment of Couples with Recurrent First Trimester and Second-Trimester Miscarriage. Green-top Guideline No. 17. [Google Scholar]
- 2.ESHRE Guideline Group on RPL. Bender Atik R, Christiansen OB, Elson J, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018:hoy004. doi: 10.1093/hropen/hoy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugiura-Ogasawara M, Suzuki S, Ozaki Y, et al. Frequency of recurrent spontaneous abortion and its influence on further marital relationship and illness: the Okazaki Cohort Study in Japan. J Obstet Gynaecol Res. 2013;39:126–131. doi: 10.1111/j.1447-0756.2012.01973.x. [DOI] [PubMed] [Google Scholar]
- 4.Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103–1111. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 5.Morita K, Ono Y, Takeshita T, et al. Risk factors and outcomes of recurrent pregnancy loss in Japan. J Obstet Gynaecol Res. 2019;45:1997–2006. doi: 10.1111/jog.14083. [DOI] [PubMed] [Google Scholar]
- 6.Ebina Y, Nishino Y, Deguchi M, Maesawa Y, Nakashima Y, Yamada H. Natural killer cell activity in women with recurrent miscarriage: etiology and pregnancy outcome. J Reprod Immunol. 2017;120:42–47. doi: 10.1016/j.jri.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Guerrero B, Hassouneh F, Delgado E, Casado JG, Tarazona RJ. Natural killer cells in recurrent miscarriage: an overview. J Reprod Immunol. 2020;142 doi: 10.1016/j.jri.2020.103209. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima A, Shima T, Inada K, Ito M, Saito S. The balance of the immune system between T cells and NK cells in miscarriage. Am J Reprod Immunol. 2012;67:304–310. doi: 10.1111/j.1600-0897.2012.01115.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamada H, Morikawa M, Furuta I, et al. Intravenous immunoglobulin treatment in women with recurrent abortions: increased cytokine levels and reduced Th1/Th2 lymphocyte ratio in peripheral blood. Am J Reprod Immunol. 2003;49:84–89. doi: 10.1034/j.1600-0897.2003.01184.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee SK, Kim JY, Lee M, Gilman-Sachs A, Kwak-Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol. 2012;67:311–318. doi: 10.1111/j.1600-0897.2012.01116.x. [DOI] [PubMed] [Google Scholar]
- 11.Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochran Database Syst Rev. 2014;10 doi: 10.1002/14651858.CD000112.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laskin CA, Bombardier C, Hannah ME, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. N Engl J Med. 1997;337:148–153. doi: 10.1056/NEJM199707173370302. [DOI] [PubMed] [Google Scholar]
- 13.de Jong PG, Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;7 doi: 10.1002/14651858.CD004734.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coomarasamy A, Williams H, Truchanowicz E, et al. A randomized trial of progesterone in women with recurrent miscarriages. N Engl J Med. 2015;373:2141–2148. doi: 10.1056/NEJMoa1504927. [DOI] [PubMed] [Google Scholar]
- 15.The German RSA/IVIG Group Intravenous immunoglobulin in the prevention of recurrent miscarriage. Br J Obstet Gynaecol. 1994;101:1072–1077. doi: 10.1111/j.1471-0528.1994.tb13584.x. [DOI] [PubMed] [Google Scholar]
- 16.Christiansen OB, Mathiesen O, Husth M., et al. Placebo-controlled trial of treatment of unexplained secondary recurrent spontaneous abortions and recurrent late spontaneous abortions with i.v. immunoglobulin. Hum Reprod. 1995;10:2690–2695. doi: 10.1093/oxfordjournals.humrep.a135769. [DOI] [PubMed] [Google Scholar]
- 17.Coulam CB, Krysa L, Stern JJ, Bustillo M. Intravenous immunoglobulin for treatment of recurrent pregnancy loss. Am J Reprod Immunol. 1995;34:333–337. doi: 10.1111/j.1600-0897.1995.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 18.Perino A, Vassiliadis A, Vucetich A., et al. Short-term therapy for recurrent abortion using intravenous immunoglobulins: results of a double-blind placebo-controlled Italian study. Hum Reprod. 1997;12:2388–2392. doi: 10.1093/humrep/12.11.2388. [DOI] [PubMed] [Google Scholar]
- 19.Stephenson MD, Dreher K, Houlihan E, Wu V. Prevention of unexplained recurrent spontaneous abortion using intravenous immunoglobulin: a prospective, randomized, double-blinded, placebo-controlled trial. Am J Reprod Immunol. 1998;39:82–88. doi: 10.1111/j.1600-0897.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 20.Jablonowska B, Selbing A, Palfi M, Ernerudh J, Kjellberg S, Lindton B. Prevention of recurrent spontaneous abortion by intravenous immunoglobulin: a double-blind placebo-controlled study. Hum Reprod. 1999;14:838–841. doi: 10.1093/humrep/14.3.838. [DOI] [PubMed] [Google Scholar]
- 21.Christiansen OB, Pedersen B, Rosgaard A, Husth M. A randomized, double-blind, placebo-controlled trial of intravenous immunoglobulin in the prevention of recurrent miscarriage: evidence for a therapeutic effect in women with secondary recurrent miscarriage. Hum Reprod. 2002;17:809–816. doi: 10.1093/humrep/17.3.809. [DOI] [PubMed] [Google Scholar]
- 22.Stephenson MD, Kutteh WH, Purkiss S, et al. Intravenous immunoglobulin and idiopathic secondary recurrent miscarriage: a multicentered randomized placebo-controlled trial. Hum Reprod. 2010;25:2203–2209. doi: 10.1093/humrep/deq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutton B, Sharma R, Fergusson D., et al. Use of intravenous immunoglobulin for treatment of recurrent miscarriage: a systematic review. BJOG. 2007;114:134–142. doi: 10.1111/j.1471-0528.2006.01201.x. [DOI] [PubMed] [Google Scholar]
- 24.Christiansen O, Larsen EC, Egerup P, Lunoee L, Egestad L, Nielsen HS. Intravenous immunoglobulin treatment for secondary recurrent miscarriage: a randomised, double-blind, placebo-controlled trial. BJOG. 2015;122:500–508. doi: 10.1111/1471-0528.13192. [DOI] [PubMed] [Google Scholar]
- 25.Yamada H, Kishida T, Kobayashi N, Kato EH, Hoshi N, Fujimoto S. Massive immunoglobulin treatment in women with four or more recurrent spontaneous primary abortions of unexplained aetiology. Hum Reprod. 1998;13:2620–2623. doi: 10.1093/humrep/13.9.2620. [DOI] [PubMed] [Google Scholar]
- 26.Yamada H, Takeda M, Maezawa Y, et al. A high dose intravenous immunoglobulin therapy for women with four or more recurrent spontaneous abortions. ISRN Obstet Gynecol. 2012;2012 doi: 10.5402/2012/512732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 28.Christiansen OB, Kolte AM, Krog MC, et al. Treatment with intravenous immunoglobulin in patients with recurrent pregnancy loss: an update. J Reprod Immunol. 2019;133:37–42. doi: 10.1016/j.jri.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. 2012;367:2015–2025. doi: 10.1056/NEJMra1009433. [DOI] [PubMed] [Google Scholar]
- 30.Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29:491–498. doi: 10.1093/intimm/dxx039. [DOI] [PubMed] [Google Scholar]
- 31.Takeda M, Yamada H, Iwabuchi K, et al. Administration of high-dose intact immunoglobulin has an anti-resorption effect in a mouse model of reproductive failure. Mol Hum Reprod. 2007;13:807–814. doi: 10.1093/molehr/gam061. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka J, Kitashoji A, Fukunaga Y, Kashihara J, Nakano A, Kamizono A. Intravenous immunoglobulin suppresses abortion relates to an increase in the CD44bright NK subset in recurrent pregnancy loss model mice. Biol Reprod. 2016;95:37. doi: 10.1095/biolreprod.116.138438. [DOI] [PubMed] [Google Scholar]
- 33.Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373:2089–2090. doi: 10.1056/NEJMc1500421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.