Figure 6. Model proposed for product release of ClpP.
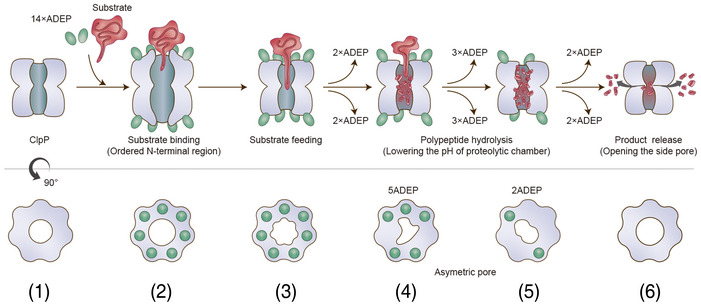
Before (state 1) and immediately after substrate binding (state 2), ClpP is in an active extended conformation, and ADEP widens the entrance pore for better substrate feeding. Our previous crystal structure of the 14 ADEP complex showed a disordered N‐terminal region (Lee et al, 2010), and our current cryo‐EM structure of the same complex demonstrates an ordered N‐terminal region. In both cases, the entrance pore is enlarged. From the extended structure (states 1 and 2) to the compressed structure (states 5 and 6), intermediate conformations were observed. Based on the solution cryo‐EM analysis, these intermediate conformations represented heterogeneous complex species of BsClpP with different numbers of ADEP molecules bound, including a 14 ADEP‐bound compact structure (state 3). Two major species among the intermediate states, 5ADE P (state 4) and 2ADEP (state 5), were crystallized and showed dimensions similar to those of previously known “compact” and “compressed” structures (Geiger et al, 2011; Lee et al, 2011; Zhang et al, 2011; Gersch et al, 2012). Both possess asymmetric entrance pores, especially 5ADEP, which is more distorted. 5ADEP is less compressed, and thus, the number and size of the side pores are fewer and smaller than those of a fully compressed structure, respectively. Apo‐BsClpP at pH 4.2 exhibits compression and larger side exit pores (state 6). The extended states 1 and 2 possess the active catalytic triad configuration (Fig 5A and B). The catalytic triad of the other states (3–6) shows an inactive and distorted configuration (Fig 5C–F). In the model, the red color of the proteolytic chamber represents the lower pH condition derived from substrate hydrolysis.
