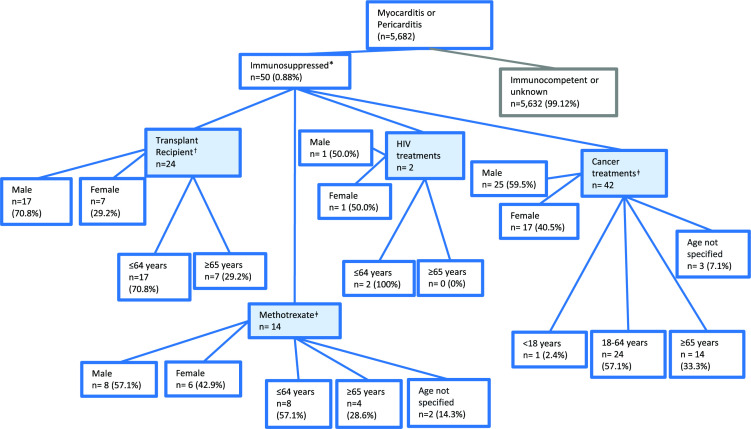
Figure 1.
Tree diagram detailing the characteristics of vaccinees submitting spontaneous reports of myocarditis and pericarditis to EudraVigilance for European Union and European Economic Area only, following COVID-19 messenger RNA (mRNA) vaccines in immunocompromised individuals. Reports of myocarditis or pericarditis were searched in the EudraVigilance database following COVID-19 mRNA vaccines, either Moderna (Spikevax) or Pfizer/BioNTech (Comirnaty) (n=50 reports). The resultant reports were further assessed for individuals that were likely to be immunosuppressed based on the following search terms (transplant medications*, HIV/AIDS treatments, EU-approved cancer therapies). Concomitant medications were used as a proxy for disease status. *Counts are not mutually exclusive; one report may contain multiple concomitant medications from different categories. †Where tacrolimus, mycophenolate mofetil, ciclosporin or prednisolone were reported as concomitant medications. ‡Methotrexate was included as a potential transplant medication, however due to its wide use in autoimmune disease this was listed independently of the treatments used as a proxy for transplantation.