Figure 5: Putative molecular determinants of subtype-selective binding at 5-HT2-type receptors.
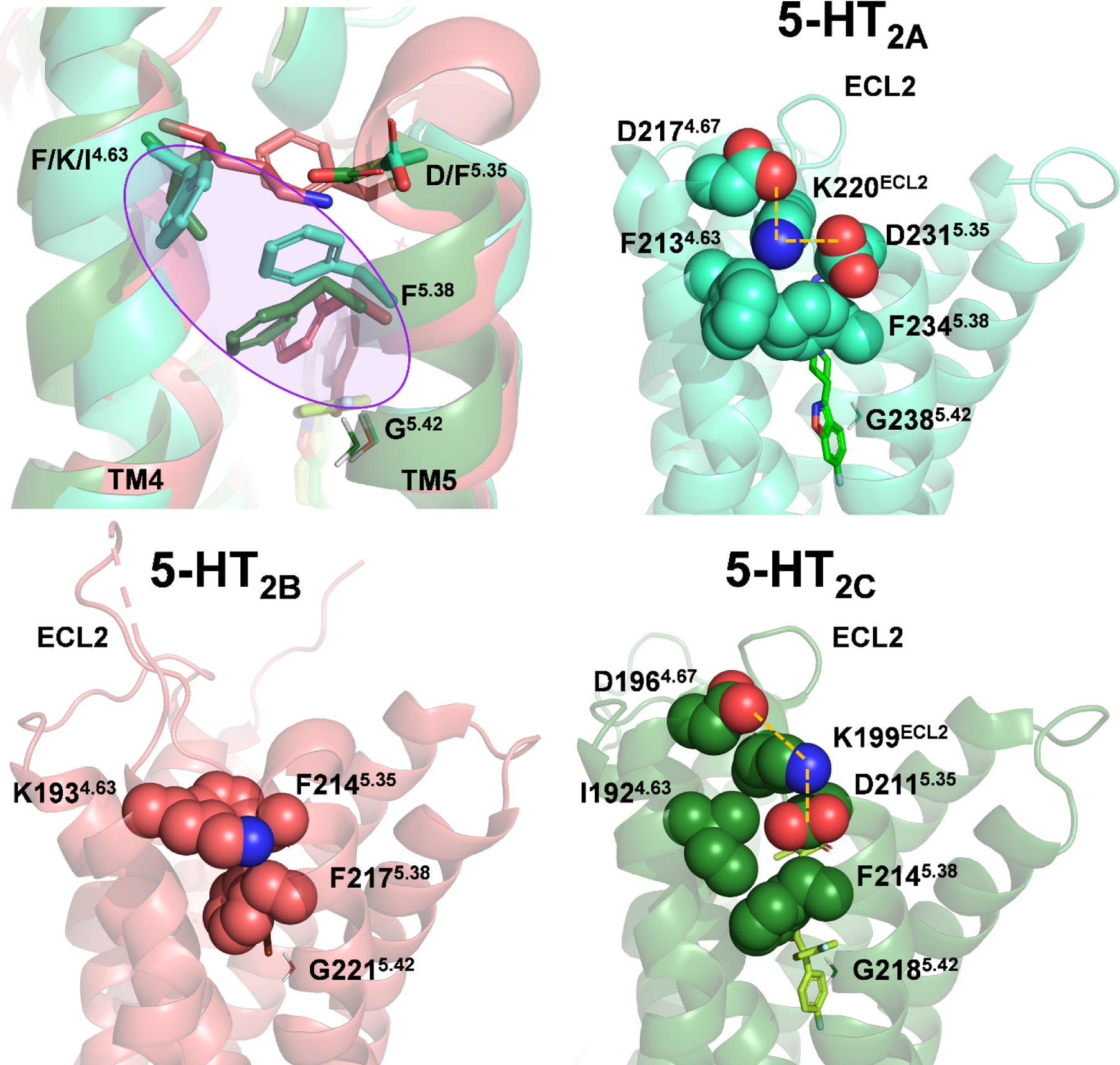
Superimposition of 5-HT2-type receptors suggests that a “side extended cavity” is formed in the 5-HT2AR (light blue color) by a unique rotamer of F5.38, making hydrophobic interactions with the non-conserved residue F4.63 (purple circle, top left). Structures of the 5-HT2AR (PDB: 6A93, top right), 5-HT2BR (PDB: 6DS0, bottom left), and 5-HT2CR (PDB: 6BQH, bottom right) indicate that ECL2 of the 5-HT2BR exhibits greater conformational freedom compared 5-HT2A and 5-HT2CRs, wherein KECL2 is electrostatically constrained (orange dashed lines). The side chain of G5.42 is shown for reference to the side cavity.
