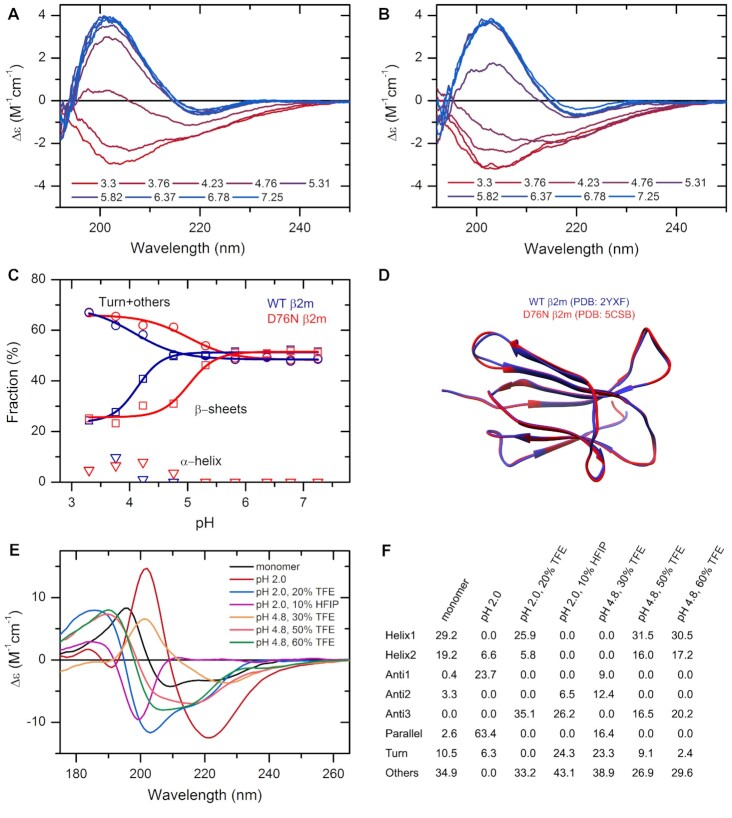
Figure 2.
The effect of environmental conditions on the protein structure studied by CD spectroscopy and analyzed by the BeStSel web server. (A, B) CD spectra of wild type (A) and D76N mutant β2m (B) were recorded at various pH values in 10 mM Na-citrate buffer at 37°C. (C) Secondary structure contents provided by BeStSel were added up as α-helix, β-sheet and turn + others. The mutant protein (red) is more sensitive to pH drop than the wild-type one (blue) and starts to loose its β-structure below pH 6.0. CD measurements were carried out on a benchtop Jasco J-1500 spectropolarimeter (1 mm pathlength, 50 nm/min scan rate, 2 sec response time, 1 nm bandwidth, accumulation: 6). (D) The two β2m variants exhibit very similar high-resolution native structure making difficult to explain the difference in the pathology. (E) Insulin can exhibit various conformations depending on the solution conditions. Its native, monomeric state is α-helical. Under nonnative conditions, such as low pH and the presence of alcohols, it forms β-structured aggregates with different β-sheet compositions as shown in (F). At pH 2.0, it forms amyloid fibrils with characteristic parallel β-sheets. These spectra were collected by SRCD at DISCO beamline in SOLEIL Synchrotron, France.