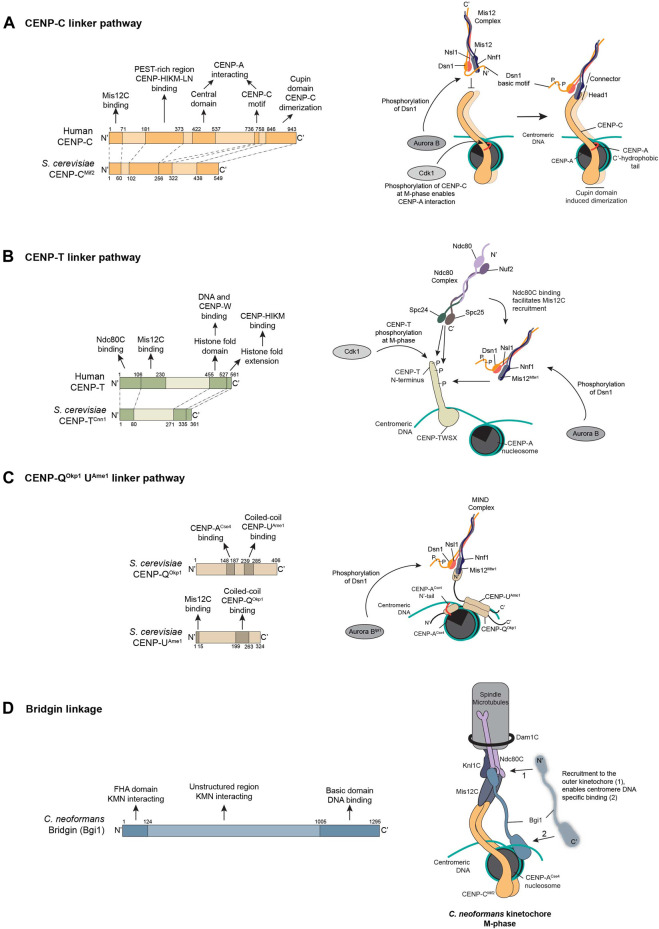
FIGURE 2.
Linker pathways connect the outer kinetochore to centromeric chromatin. (A) The CENP-C linker pathway originates through the interactions of CENP-C with the C-terminal hydrophobic tail of CENP-A at the inner kinetochore. Subsequently, CENP-C through its N-terminal motif interacts with the Mis12-Nnf1 head of the Mis12C. This interaction is weakened/inhibited by the Dsn1 basic motif that binds to Mis12 and diminishes interaction with CENP-C in its unphosphorylated form. Aurora B-dependent phosphorylation alleviates this autoinhibition. (B) CENP-T complex interacts with centromeric linker DNA through a nucleosome-like structure formed by the histone-fold domains of CENP-T-W-S-X. At the N-terminus, in the human CENP-T, two Ndc80C recruitment sites exist which is under the control of Cdk1 phosphorylation. This Ndc80C binding subsequently facilitates the recruitment of the Mis12C. The phosphorylated form of Mis12C by Aurora B preferentially binds to CENP-T. (C) CENP-QOkp1-UAme1 has been described to serve as a linker pathway in budding yeast exclusively. While CENP-QOkp1 interacts with the CENP-ACse4 N-terminal tail, CENP-QAme1 has been described to interact with the Mis12Mtw1-Nnf1 head similar to CENP-CMif2 ensuring the recruitment of the Mis12CMIND. (D) In C. neoformans, CENP-CMif2 is the only conventional linker pathway described. Interestingly, a Ki-67-like protein named bridgin (Bgi1) was identified which is recruited to the outer kinetochore by the KMN network. This kinetochore-specific recruitment facilitates Bgi1 to subsequently interact with centromeric chromatin through its basic C-terminal motif. Thus generating a linkage between the outer kinetochore and centromeric chromatin.