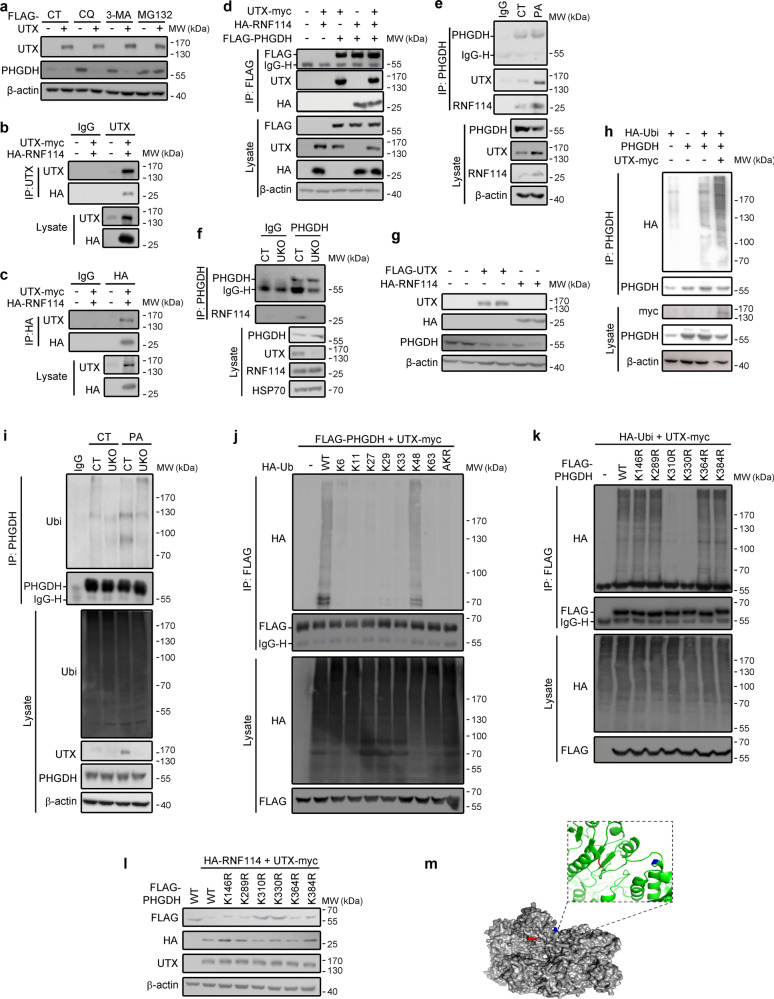
Fig. 8. UTX regulated PHGDH degradation in cultured cells.
a Effects of CQ, 3-MA and MG132 on UTX-mediated destabilization of PHGDH in HK-2 cells. b, c Co-immunoprecipitation by anti-UTX (b) or anti-HA (c) in HEK293T cells transfected with the indicated plasmids. d Co-immunoprecipitation of UTX, RNF114 and PHGDH in HEK293T cells transfected with indicated plasmids. β-actin serves as the loading control. e Endogenous co-immunoprecipitation of UTX, RNF114 and PHGDH with or without PA treatment in HK-2 cells. β-actin serves as the loading control. f Co-immunoprecipitation of endogenous PHGDH and RNF114 by anti-PHGDH in UTX KO or control HK-2 cells. HSP70 serves as the loading control. g Representative Western blots of UTX, RNF114 (HA), and PHGDH in HK-2 cells transfected with indicated plasmids. h Immunoblot analysis of the anti-PHGDH immunoprecipitates from HEK293T cells transfected with indicated plasmids and treated with MG132. i UTX knockout reduced ubiquitination of endogenous PHGDH in the HK-2 cells treated with MG132. β-actin serves as the loading control. j, k Immunoblot analysis of the anti-FLAG immunoprecipitate from HEK293T cells transfected with indicated plasmids and treated with MG132. K6, K11, K27, K29, K33, K48 and K63, ubiquitin mutants with respective lysine substituted by arginine; AKR, a ubiquitin mutant with all seven indicated lysines substituted by arginine; K146R, K289R, K310R, K330R, K364R, K384R, PHGDH mutants with respective lysine substituted by arginine. l Representative Western blots of UTX, HA, and FLAG in HK-2 cells transfected with indicated plasmids. m Crystal structure of PHGDH built by homology modeling. a–l Each experiment was repeated for at least three times with representative results shown. Source data are provided in the Source Data file.