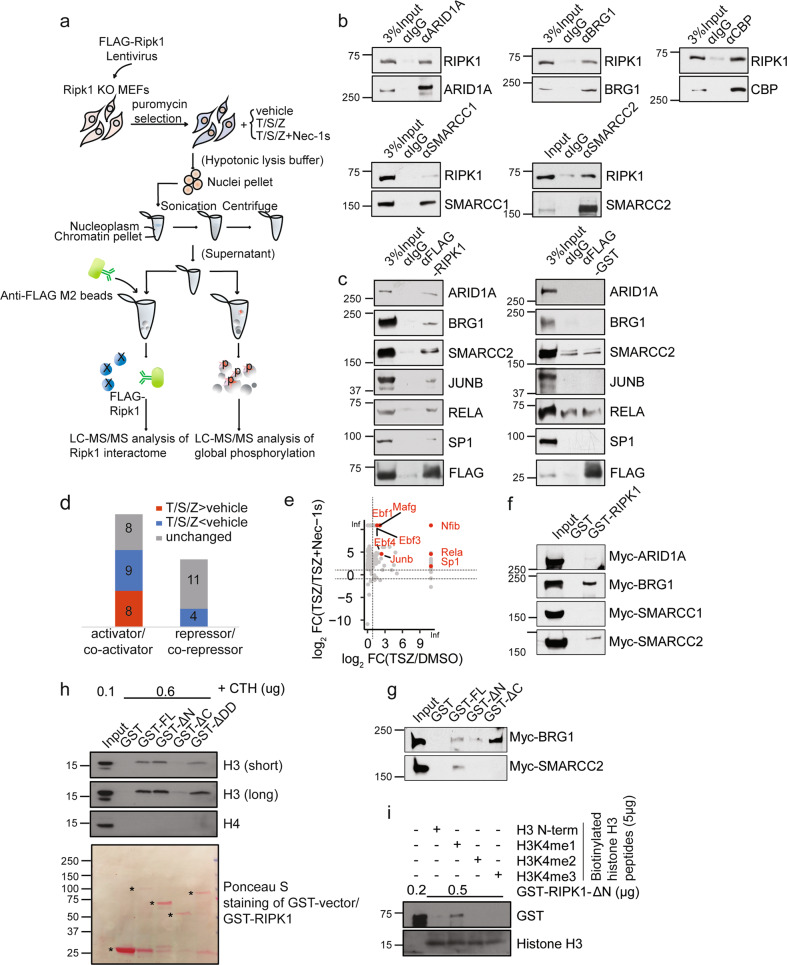
Fig. 2. RIPK1 is a transcriptional co-activator associated with multiple transcription factors and BAF complex.
a Schematic illustration of the quantitative mass spectrometry approach to identify the RIPK1 nuclear interactome and phosphoproteome. b Cell lysates from wild-type MEFs were immunoprecipitated with antibodies against the indicated proteins followed by western blotting with antibody against RIPK1. c Ripk1−/− MEFs were infected with FLAG-Ripk1 or FLAG-GST lentivirus. Nuclear lysates were immunoprecipitated with anti-FLAG M2 beads or IgG followed by protein G beads, eluted and followed by western blotting with antibodies against the indicated proteins. d The intensities of proteins detected in RIPK1 nuclear interactome under different treatments were compared and the RIPK1-interactive proteins were divided into three groups: T/S/Z > vehicle (red), T/S/Z < vehicle (blue) and unchanged (gray). Numbers of the transcriptional activators/co-activators and repressors/co-repressors from the three groups detected to interact with RIPK1 are shown. e Plot showing log2(TSZ/Vehicle) ratio of intensities detected in nuclear RIPK1 interactome on x axis and log2(TSZ/(TSZ + Nec-1s)) ratio of intensities on y axis. Active transcription factors which were detected to interact with activated RIPK1 are labeled. f The result of GST pull-down assays with Myc-ARID1A, Myc-BRG1, Myc-SMARCC1, Myc-SMARCC2 synthesized by in vitro transcription/translation, and GST, GST-RIPK1 purified from E. coli. g The result of GST pull-down assays with Myc-BRG1, Myc-SMARCC2 synthesized by in vitro transcription/translation, and GST, GST-RIPK1-full length (FL), GST-RIPK1-ΔN, GST-RIPK1-ΔC purified from E. coli. h In vitro pull-down of calf thymus histones (CTH) with indicated purified GST-fused FL or truncations of human RIPK1(ΔN, ΔC or ΔDD) was determined by western blotting with antibodies against histone H3 and H4. RIPK1-ΔDD, RIPK1 with the death domain deleted. i In vitro peptide pull-down assays. Biotinylated histone H3 peptides with mono-, di-, tri-methylation (5 µg), or without methylation on H3K4 were used to pull down purified GST-RIPK1-ΔN. The histone binding preferences were determined by western blotting with the antibody against GST tag.