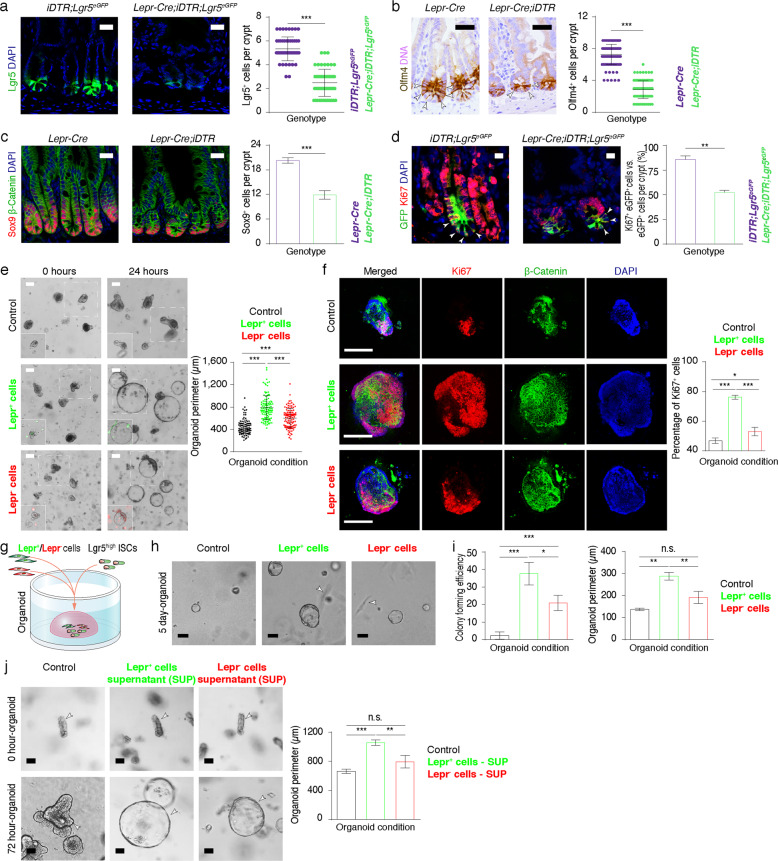
Fig. 3. Lepr+ MCs sustain the proliferation of ISCs.
a Immunofluorescence of GFP (Lgr5-GFP) in jejunum from iDTR;Lgr5-eGFP-CreERT2 (iDTR;Lgr5eGFP) and Lepr-Cre;iDTR;Lgr5-eGFP-CreERT2 (Lepr-Cre;iDTR;Lgr5eGFP) mice after 10 days of consecutive DT treatment. Quantification of Lgr5+ cells per crypt under both conditions (n = 3). Scale bar, 25 μm. b, c Immunostaining for Olfm4 and Sox9/β-Catenin in the jejunum from Lepr-Cre and Lepr-Cre;iDTR mice after 10 days of consecutive DT treatment. Quantification of Olfm4+ cells and Sox9+ cells per crypt under both conditions (n = 3). Scale bar, 25 μm. d Double immunofluorescence for GFP and Ki67 in jejunum from iDTR;Lgr5eGFP and Lepr-Cre;iDTR;Lgr5eGFP mice after 10 days of consecutive DT treatment. Quantification of percentage of Ki67+Lgr5+ cells versus Lgr5+ cells under both conditions (n = 3). Scale bar, 10 μm. e Representative images and quantification of intestinal organoids co-cultured with vehicle control, Lepr+ cells or Lepr– cells at indicated time points after seeding. Crypts were seeded at the same initial density. High-resolution images are shown in the left bottom corner as insets, in which Lepr– or Lepr+ cells can be observed. n = 108 organoids in each group. Scale bar, 100 μm. f Immunofluorescence for Ki67 and β-Catenin for organoids 24 h after seeding in e. Quantification of percentage of Ki67+ cells under the indicated conditions. n = 30 organoids in control group, n = 40 organoids in experimental groups co-cultured with Lepr– or Lepr+ cells. Scale bar, 100 μm. g Schematic diagram showing the co-culture system of Lgr5high single cells with Lepr+ or Lepr– cells. h Representative images of spheroids derived from Lgr5high cells under distinct culture conditions for control, Lepr+ or Lepr– cells after 5 days of co-culture. n = 2 biologically independent experiments, 3 technical replicates each. Scale bar, 20 μm. i Quantification of the colony-forming efficiency and perimeter of Lgr5high cell-derived spheroids in h. j Representative images of intestinal organoids cultured with the supernatants from Lepr+ or Lepr– cells at the indicated time points after seeding. Crypts were seeded at the same initial density. Quantification of the perimeter of organoids at 72 h. n = 2 biologically independent experiments, 3 technical replicates each. Scale bar, 50 μm. Values in the graphs represent means ± SD. Unpaired Student’s t-test was used for calculating P values in a–f, i and j. *P < 0.05; **P < 0.01; ***P < 0.001.