FIGURE 2.
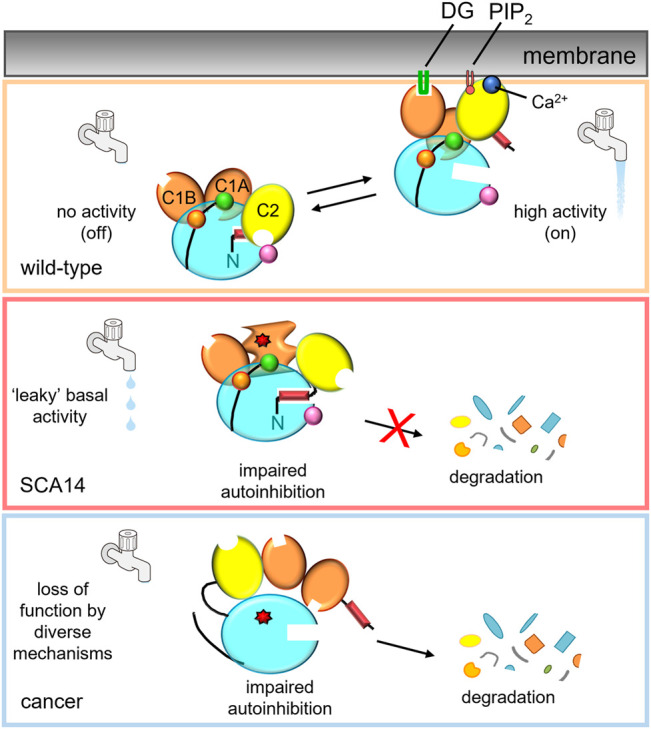
PKCγ mutations in disease lead to differing effects on kinase activity.Top: In the absence of second messengers, wild-type PKCγ adopts an autoinhibited conformation, in which no signaling occurs (water faucet is “off”). In the presence of Ca2+ and DG, wild-type PKCγ adopts an open conformation and is activated (water faucet is “on).Middle: Mutations in SCA14 lead to impaired autoinhibition of PKCγ resulting in “leaky activity”; mutations in the C1 domains protect PKC from down regulation, evading quality control degradation of the impaired PKC. This species can be further activated by second messenger binding for some, but not all, SCA14 mutations (not shown). Bottom: Mutations in cancer lead to loss of PKC function by diverse mechanisms. One common mechanism is by impairing autoinhibition, resulting in the dephosphorylation and degradation of PKC. Mutant PKCγ can also act in a dominant negative manner to suppress signaling by other PKC isozymes.
