Abstract
Objective:
To evaluate the utility and safety of tracheostomy for patients with respiratory failure from COIVD-19 and describe patient clinical characteristics and process of management.
Methods:
Case series of the first 24 COVID-19 patients who underwent tracheostomy at our institution, a single-center tertiary care community hospital intensive care/ventilator weaning unit. The patients all had respiratory failure from COVID-19 and required endotracheal intubation and mechanical ventilation. Outcomes reviewed include mortality, percent discharged, percent liberated from mechanical ventilation, percent decannulated, time from tracheostomy to ventilator liberation and discharge, and number of staff infected with COVID-19 during tracheostomy and management.
Results:
Of the 24 patients who underwent tracheostomy, 21 (88%) of 24 survived. Twenty (83%) were liberated from mechanical ventilation, and 19 (79%) were discharged. Fourteen (74%) of the discharged had been decannulated. The average (± SD) time from tracheostomy to ventilator liberation was 9 ± 4.3 days and from tracheostomy to discharge 21 ± 9 days. All discharged patients had been liberated from mechanical ventilation. No health care workers became infected with COVID-19 during the procedure or subsequent patient management.
Conclusion:
Patients with respiratory failure from COVID-19 who underwent tracheostomy had a high likelihood of being liberated from mechanical ventilation and discharged. Tracheostomy and subsequent ventilator weaning management can be performed safely. Tracheostomy allowed for decompression of higher acuity medical units in a safe and effective manner.
Keywords: COVID-19, tracheostomy, outcomes, safety, respiratory failure, ventilator weaning
Background
The COVID-19 respiratory virus has triggered a global pandemic leading to increased intensive care unit (ICU) admissions for respiratory failure and acute respiratory distress syndrome (ARDS).1 Our understanding of the management of these patients is still evolving, but patients in respiratory failure from COVID-19 appear to have a protracted course.2,3 Traditionally, patients who are clinically stable and require prolonged mechanical ventilation have tracheostomy performed, typically after 1 to 3 weeks of endotracheal intubation.4,5 The benefits of tracheostomy include decreased sedation, improvement in work of breathing,6,7 improved pulmonary toilet and oral hygiene,8 facilitation of physical therapy, decreased rates of subglottic stenosis, and possibly reductions in ventilator associated pneumonia.9 Given the aerosolization of virus that occurs when performing procedures involving the airway, there has been concern over performing tracheostomies in COVID-19 patients.10 There is also little data on outcomes of patients with COVID-19 who have had tracheostomies. We present a case series of the first 24 patients with respiratory failure due to COVID-19 who had tracheostomies performed at our institution.
Materials and Methods
This study was declared exempt from review by the institutional review board of White Plains Hospital. White Plains Hospital is a tertiary suburban community hospital situated in Westchester County outside of New York City. During the COVID-19 pandemic, the hospital created a specialized ward for COVID-19 patients who were being weaned from mechanical ventilation after tracheostomy. A multidisciplinary team consisting of an intensivist, hospitalist, otolaryngologist, nurse, respiratory and physical therapist, nutritionist, and wound care specialist performed daily morning rounds. Dedicated education to the care team was provided during its formation by ICU physician and nursing leaders. The nursing care team was set up to have a 1:3 nurse to patient ratio to provide the necessary care hours to supplement intense physical therapy that occurred twice a day as well as frequent respiratory assessments. The plan of care was standardized and multifaceted. It included complete cessation of sedation after tracheostomy. Shorter acting analgesics were given as needed in lieu of drips. Diuresis was targeted to euvolemia. Ventilator weaning was aggressive, with conversion to the lowest level of pressure support ventilation (PSV) tolerated beginning no later than the day after tracheostomy. PSV was then maintained 24 hours a day if tolerated, with incremental adjustments as needed. If a patient required placement back on assist control, PSV was reinstituted later that day but no later than the subsequent morning. Once capable of tolerating unassisted breathing, patients were disconnected from the ventilator, and a heat moisture exchanger with viral filter was used in lieu of a tracheostomy collar to reduce aerosolization of tracheobronchial secretions.
Patients were evaluated daily for ability to participate in physical therapy and received a range of therapy from passive range of motion to unassisted ambulation. Family support via videophone, both during physical therapy sessions and throughout the stay, was used to provide additional stimulation and motivation.
Definitions
Maximum ventilator settings were the peak fraction inspired oxygen (FIO2) and positive end-expiratory pressure (PEEP) and, to qualify, had to occur at least 12 hours out from intubation and for at least 2 hours in a row. Data on averages and ranges account for patients with a defined outcome at the time of submission.
Tracheostomy Procedure
The surgery was completed by a 2-surgeon team, both in powered air-purifying respirators (PAPRs), with a surgical head light secured to one of the helmets. Each surgeon additionally donned an N95 mask, booties, cap, and 2 surgical gowns, one beneath the PAPR, and another over the PAPR. The scrub and circulating nurses were asked to wear a PAPR as well. If the PAPRs were not available, then the scrub nurse would set up the case and depart prior to skin incision to decrease risk of exposure.
Tracheostomy was performed in similar fashion to a standard tracheostomy with a few minor adjustments meant to protect the staff and surgical team from aerosolized viral particles. A horizontal skin incision was performed and carried down to the strap musculature. The strap musculature was divided in midline to expose the thyroid isthmus. Care was taken in each case to elevate the isthmus off the anterior tracheal wall and divide the tissue with electrocautery followed by a suture ligature. This decreased the risk of postoperative oozing from the isthmus, which would have necessitated treatment and increased exposure risk postoperatively. In addition, many patients were on anticoagulation protocols which increased the risk of postoperative bleeding from the thyroid bed and open tracheostomy incision.
After division of the thyroid isthmus, preparation for a Bjork flap was undertaken. The anesthesiologist was then asked to hold ventilation, deflate the cuff, advance the tube toward the carina, reinflate, and commence ventilation. The advanced endotracheal tube decreased the risk of injury to the balloon of the tube which would create an air leak and increase aerosolized viral particles. The tracheotomy was then performed horizontally. The tracheal ring inferior to the tracheotomy was divided bilaterally to create an inferiorly based hinge flap. The flap was secured to the inferior aspect of the skin incision in typical Bjork fashion.
Care was taken to select an appropriate tracheostomy tube. Our preference was to place a larger tracheostomy tube in order to decrease the risk of a cuff leak and to decrease the risk of mucous plugging. Additionally, if patients were obese with significant soft tissue overlying the anterior tracheal wall, a proximally extended endotracheal tubes was placed. The procedure was completed by once again holding ventilation, deflating the endotracheal tube cuff, delivering the cuff proximally, and placing the appropriate tracheostomy tube. The tracheostomy tube was inflated and then anesthesia was directed to commence ventilation. After confirming CO2 exchange, the endotracheal tube was removed. The tracheostomy was secured with 4 point silk sutures and a cotton tie circumferentially.
Results
Five (21%) of the patients were female (Table 1). The average age was 61.1 years old (range 36-76). The most common pre-admission comorbidities were hypertension (58%), diabetes (42%), obesity (33%), and cardiovascular disease (21%). Four patients had no history of medical problems. Twenty-one of 24 (88%) developed acute kidney injury, 9 (43%) of whom required dialysis. Twenty-three of 24 (all aside from one who could not be evaluated due to mental status) developed ICU-acquired weakness (weakness secondary to critical illness polyneuropathy, myopathy, or both). Eleven (46%) patients developed shock, 3 (13%) patients had strokes, and 2 (8%) had DVTs. Other complications included bacterial superinfection, atrial fibrillation, cardiac arrest, and gastrointestinal hemorrhage. There was limited evaluation of pulmonary embolism and NSTEMI. All patients had periods of encephalopathy, for which the causes were multifactorial.
Table 1.
Patient Characteristics, Complications, and Outcomes.
| Characteristic | Number (%) |
|---|---|
| Age (average; range) | 24 (61.1; 36-76) |
| Female gender | 5 (21) |
| Comorbidities | |
| Hypertension | 14 (58) |
| Diabetes | 10 (42) |
| Obesity | 8 (33) |
| Cardiovascular disease | 5 (21) |
| CKD | 2 (8) |
| No medical history | 4 (16) |
| Complications | |
| AKI | 21 (88) |
| AKI requiring dialysis | 9 (38) |
| ICUAW | 23 (96) |
| Shock | 11 (46) |
| Stroke | 3 (13) |
| DVT | 2 (8) |
| Outcomes | |
| Survived | 21 (88) |
| Liberated | 20 (83) |
| Discharged | 19 (79) |
| Decannulated | 16 (67) |
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; DVT, deep vein thrombosis; ICUAW, intensive care unit acquired weakness.
At the time of submission, 21 (88%) of 24 patients had survived. Twenty (83%) had been liberated from the ventilator, and 19 (79%) had been discharged (Table 2). Fourteen (74%) of the discharged had been decannulated, with an additional patient decannulated and awaiting discharge and another having been decannulated and subsequently not survived. Maximum ventilator settings prior to tracheostomy were an average of 89% FIO2 and 12 cm H2O PEEP and ranged from 60% to 100% FIO2 and 5 to 16 cm H2O PEEP (Table 3). At the time of tracheostomy, the average duration of mechanical ventilation was 18.6 days, with a range of 14 to 28 days. The average number of days from tracheostomy to discontinuation of the ventilator was 9 (range 2-20) for an average total time on the ventilator of 27.6 days. The average time from tracheostomy to hospital discharge was 20.9 days (range 10-45). Total hospital length of stay averaged 43.5 days.
Table 2.
Discharge Data.
| Discharge destination | Number (% total discharge) |
|---|---|
| Acute rehabilitation | 9 (47) |
| Subacute rehabilitation | 6 (32) |
| Long-term acute care hospital | 1 (5) |
| Home | 3 (16) |
Table 3.
Ventilator and Length of Stay Data.
| Ventilator parameter | Average settings (range) |
|---|---|
| Maximum FIO2 | 89% (60%-100%) |
| Maximum PEEP | 12 (5-16 cm H2O) |
| FIO2 at time of tracheostomy | 40% (30%-60%) |
| PEEP at time of tracheostomy | 5.7 (5-10 cm H2O) |
| Tracheostomy intervals | Average days (range) |
| Admission to tracheostomy | 22.6 (15-31) |
| Intubation to tracheostomy | 18.6 (14-28) |
| Tracheostomy to liberation (survivors) | 9 (2-20) |
| Tracheostomy to discharge (survivors) | 20.9 (13-45) |
Abbreviations: FIO2, fraction inspired oxygen; PEEP, positive end-expiratory pressure.
None of the care providers including hospitalists, intensivists, surgeons, anesthesiologists, nurses, physician assistants, respiratory therapists, and physical therapists contracted COVID-19 during the management of these patients.
Discussion
In this case series, we reviewed the first 24 patients with respiratory failure on mechanical ventilation due to COVID-19 who underwent tracheostomy. Notably, the vast majority of our patients were able to be weaned off the ventilator and discharged from the hospital. Historically, outcomes for tracheostomy patients with an admitting diagnosis of respiratory failure are mixed, with a significant portion of patients remaining ventilator-dependent at discharge.11,12 In our series, 79% of patients were discharged from the hospital, all of whom had been liberated from mechanical ventilation and 74% of whom were decannulated. This likely reflects the overall baseline level of health of our COVID-19 patients. Most of them were physically independent, without preexisting lung disease, and had an average age of 61. Tracheostomy in medical ICUs is often provided for older patients with preexisting lung disease, multiple comorbidities, and poor baseline health predating their respiratory failure.12,13 Other possible contributors to the outcome include having a dedicated weaning unit with direct intensivist involvement,14 the multidisciplinary approach to management,15 and an aggressive approach to ventilator weaning and cessation of sedation. In particular, the availability of an around-the-clock intensivist facilitated continuation of weaning during night hours, when a patient might otherwise have been placed on assist control as a default setting. The high rate of ventilator liberation may also reflect the smaller sample size of patients in this study. In an earlier and larger study by Chao et al involving 53 COVID-19 patients who underwent tracheostomy, 57% were liberated from mechanical ventilation.16 At the time of submission, tracheostomy had been performed on 15% of our patients who required mechanical ventilation for COVID-19 pneumonia. This subgroup accounted for 40% of the total COVID-19 patients liberated from mechanical ventilation.
While the initial primary insult to our patients was pulmonary, the indication for tracheostomy appeared to predominantly be ICU acquired weakness. The patients almost universally had some level of moderate to severe weakness as determined by the physical medicine and rehabilitation physician on the team. While incompletely resolved pneumonia and poor lung compliance also contributed, at time of tracheostomy most patients had FIO2 and PEEP requirements that could have easily been accommodated via noninvasive means, yet could still not tolerate spontaneous breathing trials on these lower levels of support. Other causes, including encephalopathy of multifactorial etiology, volume overload, and in some cases equipment failure, also contributed. Notably, 9% of our intubated patients required endotracheal tube exchanges for occlusion, and at the time of tracheostomy, we found a number of patients with narrow endotracheal tube lumens from extensive precipitated secretions (Figure 1; Video 1). However, these latter causes were relatively quickly reversible, yet the average time from tracheostomy to liberation from mechanical ventilation was 9 days. This points to an imbalance in the strength/load ratio of the respiratory system, with the work required to breathe being greater than the body’s ability to accommodate.
Figure 1.
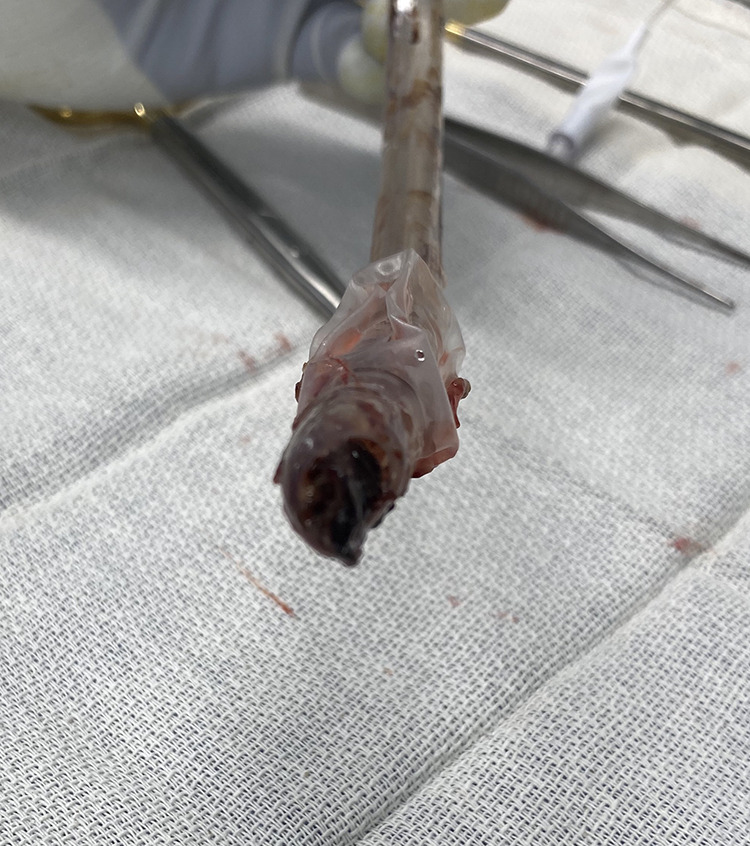
Obstructed endotracheal tube due to precipitated secretions.
There has been concern regarding the risk of COVID-19 transmission from patients on mechanical ventilation to providers, both during general medical management and during the performance of procedures that generate aerosols, for example, tracheostomy. In our series, none of the staff members involved in either the tracheostomy procedure or involved in administering medical care on the weaning unit became infected with COVID-19. This provides support to the protective effects of a protocolized approach to managing these patients’ airways and using appropriate personal protective equipment. This is also a reflection of where these patients are in their infectious course. By the time patients had tracheostomy performed, they had been on the ventilator for an average of 18.6 days, had been admitted for an average of 22.6 days, and combined with the time from exposure to hospital admission,17,18 it had likely been well over 4 weeks since becoming infected.
Given the results we have seen with these patients, we believe that tracheostomy was an integral part of the management plan for our COVID-19 patients with respiratory failure who required prolonged mechanical ventilation. While not all patients with ARDS due to COVID-19 will be candidates for tracheostomy, for the ones who do meet criteria, it was a finite intervention in their path to recovery. Providers should anticipate a prolonged hospital course, but nonetheless one with a high probability of discharge off of mechanical ventilation. This case series shows that both the tracheostomy procedure as well as the subsequent management on a weaning unit can be performed safely and effectively. In addition to providing a conduit for liberating patients from the ventilator, tracheostomy allowed for decompression of the higher level ICUs in the hospital during a time of limited resources and high volume. This freed ICU beds for patients in the earlier, more acute phase of illness which allowed for better utilization of resources without compromising patient care.
Acknowledgments
The authors would like to thank the staff of the 4I weaning unit for the dedication, compassion, and courage they have displayed throughout the pandemic.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: John J. Cardasis  https://orcid.org/0000-0002-8524-2142
https://orcid.org/0000-0002-8524-2142
Supplemental Material: Supplemental material for this article is available online.
Reference
- 1.Ezekiel J, Govind P, Ross U, et al. Fair allocation of scarce medical resources in the time of COVID-19. N EngL J Med. 2020;382(21):2049–2055. [DOI] [PubMed] [Google Scholar]
- 2.Cummings M, Baldwin M, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argenziano M, Bruce S, Slater C, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esteban A, Anzueto A, Alia I, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. 2000;161(5):1450–1458. [DOI] [PubMed] [Google Scholar]
- 5.Rana S, Pendem S, Pogodzinski M, Hubmayer R, Gajic O. Tracheostomy in critically ill patients. Mayo Clin Proc. 2005;80(12):1632–1638. [DOI] [PubMed] [Google Scholar]
- 6.Davis K, Campbell R, Johannigman J, Valente J, Branson R. Changes in respiratory mechanics after tracheostomy. Arch SurgArch Surg. 1999;134(1):59–62. [DOI] [PubMed] [Google Scholar]
- 7.Diehl J, Atrous S, Touchard D, Lemaire F, Brochard L. Changes in the work of breathing induced by tracheotomy in ventilator-dependent patients. Am J Respir Crit Care Med. 1999;159(2):383–388. [DOI] [PubMed] [Google Scholar]
- 8.Wood D. Tracheostomy. Chest Surg Clin N Am. 1996;6(4):749–764. [PubMed] [Google Scholar]
- 9.Nseir S, Di Pompeo E, Jozefowicz E, et al. Relationship between tracheotomy and ventilator-associated pneumonia: a case control study. Eur Respir J. 2007;30(2):314–320. [DOI] [PubMed] [Google Scholar]
- 10.Michette C, Burlew C, Bulger E, Davis K, Spain D. Performing tracheostomy during the Covid-19 pandemic: guidance and recommendations from the critical care and acute care surgery committees of the American Association for the surgery of trauma. BMJ 2020;5(1):e000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engoren M, Arslanian-Engoren C, Fenn-Burderer N. Hospital and long-term outcome after tracheostomy for respiratory failure. Chest 2004;125(1):220–227. [DOI] [PubMed] [Google Scholar]
- 12.Marchese S, Corrado A, Scala R, et al. Tracheostomy in patients with long term mechanical ventilation: a survey. Respir Med. 2010;104(5):749–753. [DOI] [PubMed] [Google Scholar]
- 13.Khammas A, Dawood M. Timing of tracheostomy in intensive care unit patients. Int Arch Otorhinolaryngol. 2018;22(4):437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tobin A, Santamaria J. An intensivist-led tracheostomy review team is associated with shorter decannulation time and length of stay: a prospective cohort study. Crit Care. 2008;12(2):R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrubba M, Turner T, Grieveson C. Multidisciplinary care for tracheostomy patients: a systematic review. Crit Care. 2009;13(6):R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao T, Harbison S, Braslow B, et al. Outcomes after tracheostomy in COVID-19 patients. Ann Surg. 2020;272(3):e181–e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Basteiro A, Chaccour C, Guinovart C, et al. Monitoring the COVID-19 epidemic in the context of widespread local transmission. Lancet Respir Med. 2020;8(5):440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]


