FIGURE 3.
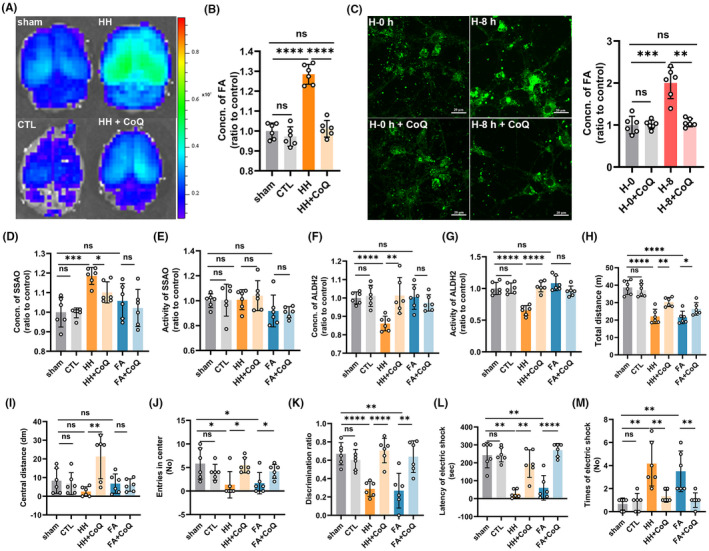
Nano‐packed coenzyme Q10 prevented formaldehyde (FA) accumulation induced by acute hypoxia and alleviated neurological deficits. (A) Cerebral FA imaging by an in vivo small animal system with NaFA probe (λex/em = 440/550 nm). (B) Cerebral FA detected by QuantiChrom FA assay kit (HH vs. HH + CoQ10, p < 0.0001). (C) Cytoplasmic FA of primary neurons detected by confocal imaging with NaFA (H‐8 h vs. H‐8 h + CoQ10, p = 0.0022). Scale bar = 20 μm. Change of the expression (HH vs. HH + CoQ10, p = 0.0141) (D) and activity (HH vs. HH + CoQ10, p = 0.5990) (E) of SSAO in the cerebrum of the mice after intervention. Change in the level (HH vs. HH + CoQ10, p = 0.0054) (F) and activity (HH vs. HH + CoQ10, p < 0.0001) (G) of ALDH2 in the cerebrum of the mice after intervention. (H–J) Neurological functions evaluated by open field test to show the total distance (HH vs. HH + CoQ10, p = 0.0020) (H), central distance (HH vs. HH + CoQ10, p = 0.0043) (I), and the frequency entering the center (HH vs. HH + CoQ10, p = 0.0325) of the mice on the first day after hypobaric hypoxia exposure (D1) (J). (K) Cognitive function evaluated by novel objective recognition (HH vs. HH + CoQ10, p < 0.0001) on D1. Learning and memory ability assessed by step down test shown as the latency to electric shock (HH vs. HH + CoQ10, p = 0.0022) (L) and times of electric shock (HH vs. HH + CoQ10, p = 0.0065) on the second day after hypobaric hypoxia exposure. (M). FA: formaldehyde; sham: unexposed control group; CTL: unexposed to HH but intragastrically administrated with CoQ10; HH: exposed to acute hypobaric hypoxia; HH + CoQ: intragastric administration with CoQ10 prior to HH exposure; FA + CoQ: intraperitoneal injection of FA and intragastric administration with CoQ10. N = 6 in each group, with three independent experiments. Data are presented as mean ± SD; ns: p > 0.05, *p < 0.05, **p <0.01, ***p < 0.001, ****p < 0.0001
