Figure 6. LLPS‐deficient CBX4 bodies are unable to suppress HIV‐1 and SUMOylate EZH2.
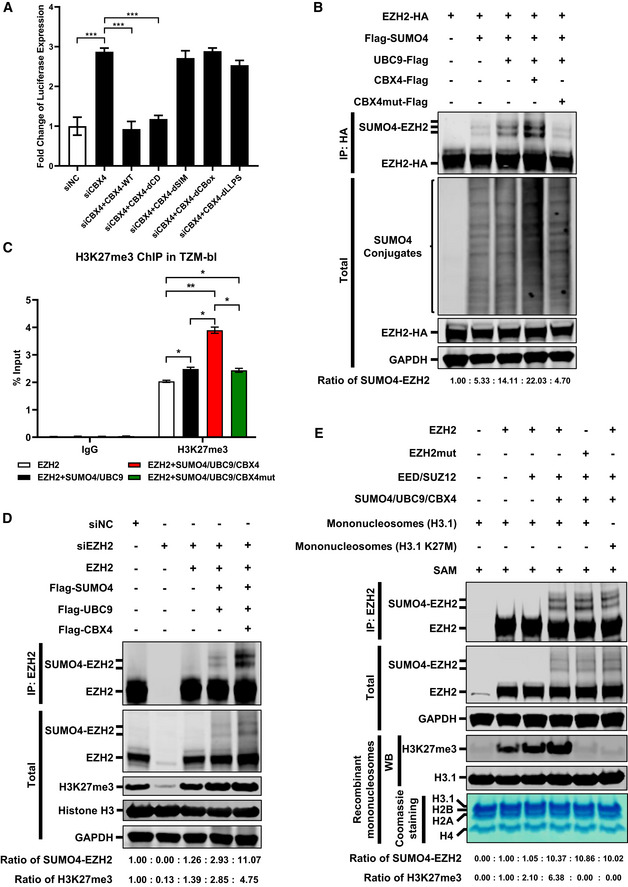
- The endogenous CBX4 was knocked down by siRNA targeting 3’UTR of CBX4 mRNA, followed by the overexpression of wild‐type CBX4 and CBX4 mutants including CBX4‐dCD, CBX4‐dSIM, CBX4‐dCBox and CBX4‐dLLPS. The expression of luciferase within each group was measured and normalized to the siNC group.
- Two micrograms (2 μg) of HA‐tagged EZH2 were co‐overexpressed with 4 μg of Flag‐tagged SUMO4, 250 ng of Flag‐tagged UBC9, 250 ng of Flag‐tagged CBX4 or CBX4mut within 6 cm dishes. The CBX4mut was the LLPS‐deficient CBX4. EZH2 was IP with anti‐HA beads. Both total and IP samples were IB with antibodies against HA, Flag and GAPDH. The expression ratios of SUMO4‐EZH2 are marked below the panel.
- Two micrograms (2 μg) of EZH2 was overexpressed in TZM‐bl cells within 6 cm dishes. In the second group, 2 μg of EZH2 was co‐overexpressed with 4 μg of SUMO4 and 250 ng of UBC9. In the third group, 2 μg of EZH2 was co‐overexpressed with 4 μg of SUMO4, 250 ng of UBC9 and 250 ng of CBX4. In the last group, 2 μg of EZH2 was co‐overexpressed with 4 μg of SUMO4, 250 ng of UBC9 and 250 ng of CBX4mut. ChIP assays with antibodies against IgG and H3K27me3 were performed for each group. Only “B” position signals are shown and normalized to input.
- The endogenous EZH2 in TZM‐bl cells within 6 cm dishes was knocked down by siRNAs targeting 3’UTR of EZH2 mRNA, followed by the overexpression of 2 μg of EZH2, 4 μg of Flag‐tagged SUMO4, 250 ng of Flag‐tagged UBC9 or 250 ng of Flag‐tagged CBX4. EZH2 was IP with anti‐EZH2 antibodies. IP samples were IB with anti‐EZH2 antibodies. Total samples were IB with anti‐EZH2, anti‐H3K27me3, anti‐Histone H3 and anti‐GAPDH antibodies. The expression ratios of SUMO4‐EZH2 and H3K27me3 are marked below the panel.
- In the first group, HEK293T cells within 6 cm dishes were transfected with empty vectors. In the second group, 3 μg of EZH2 was overexpressed. In the third group, 3 μg of EZH2 was co‐overexpressed with 2 μg of EED and 2 μg of SUZ12. In the fourth and sixth group, 3 μg of EZH2 was co‐overexpressed with 2 μg of EED, 2 μg of SUZ12, 2 μg of SUMO4, 500 ng of UBC9 and 2 μg of CBX4. In the fifth group, 3 μg of EZH2mut (Y731D) was co‐overexpressed with 2 μg of EED, 2 μg of SUZ12, 2 μg of SUMO4, 500 ng of UBC9 and 2 μg of CBX4. Forty‐eight hours post transfection, EZH2 and EZH2mut were IP with anti‐EZH2, followed by the incubation with 2 μg of mononucleosomes (H3.1) and 20 μM of the cofactor S‐adenosyl‐L‐methionine (SAM) to measure the methyltransferase activity of EZH2. The IP reaction in the sixth group was incubated with 2 μg of mononucleosomes (H3.1 K27M) and 20 μM of SAM. Both total and IP samples were IB with anti‐EZH2, GAPDH, H3K27me3 and H3.1 antibodies. The purities of recombinant mononucleosomes which included H3.1, H2B, H2A and H4 were verified by Coomassie blue staining. The expression ratios of SUMO4‐EZH2 and H3K27me3 are marked below the panel.
Data information: Data in (A) and (C) are presented as mean ± SEM in biological triplicate. P‐values in (A) were calculated by one‐way ANOVA with Tukey's multiple comparisons test. P‐values in (C) were calculated by two‐way ANOVA with Tukey's multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001.
