Abstract
Patient: Male, 46-year-old
Final Diagnosis: Ankylosing spondylitis
Symptoms: Low back pain
Medication:—
Clinical Procedure: —
Specialty: Rehabilitation • Rheumatology
Objective:
Rare coexistence of disease or pathology
Background:
Ankylosing spondylitis (AS) is an immune-mediated chronic inflammatory condition grouped under spondylo-arthritis (SpA), which is an umbrella term for a group of interrelated inflammatory rheumatic conditions with characteristic radiographic findings such as erosions and ankylosis of the sacroiliac joint. Unfortunately, there is an average delay of 8–9 years between the onset of the symptoms and diagnosis due to infrequent consideration of this disease in the differential diagnosis of patients with low back pain and unusual or incomplete presenting clinical symptoms.
Case Report:
We describe the case of a 37-year-old male patient with no significant past medical history and surgical history of bilateral hip arthroplasty secondary to idiopathic aseptic necrosis of the bilateral femoral head and bilateral rotator cuff repaired surgery due to multiple motor vehicle accidents (MVA) with a chief concern of chronic low back pain. In this case of ankylosing spondylitis presenting with low back pain and radicular symptoms, his symptoms were resistant to multiple opioid medications, trigger point injections, and epidural steroid injections. Initiation of adalimumab subsequently relieved the patient’s symptoms and restored his ability to perform daily activities.
Conclusions:
This is an unusual presentation of AS with radiographic evidence of bilateral sacroiliitis. The neurological manifestations in AS are not uncommon, and they can occur during the quiescent stage of the disease. It should be emphasized that early diagnosis is essential to prevent progression of the disease and avoid unnecessary treatment for the patient.
Keywords: Adalimumab; HLA-B27 Antigen; Radiculopathy; Sacroiliitis; Spondylitis, Ankylosing
Background
Ankylosing spondylitis (AS) is an immune-mediated chronic inflammatory condition grouped under spondyloarthritis (SpA) with a prevalence of 0.1% to 1.4% worldwide, and males are affected more frequently than females [1,2]. Spondyloarthritis (SpA) is an umbrella term for a group of interrelated inflammatory rheumatic conditions that include sacroiliitis, spondylitis, peripheral arthritis, enthesitis, dactylitis, acute anterior uveitis, associated psoriasis or inflammatory bowel disease, presence of HLA-B27, and no association with rheumatoid factor [3]. The SpA can be subdivided into axial SpA or ankylosing spondylitis (predominant symptoms of spine and sacroiliac joints) and peripheral SpA (predominant symptoms of peripheral arthritis, enthesitis, and or dactylitis) [3,4].
An early diagnosis of AS is critical because effective treatments are available, and they are more efficacious if used in the early stage of the disease [5–7]. Back pain is also the first symptom and most frequent manifestation in the AS [7,8]. Additionally, bilateral sacroiliitis is the hallmark of AS, and detecting radio-graphic sacroiliitis is pivotal for diagnosing AS [9,10]. Moreover, SpA accounts for only about 5% of chronic back pain, which is an extremely frequent symptom in pain management facilities and is widespread in the general population [11,12]. Therefore, subsequent referral to the rheumatologist of those patients with back pain with a higher probability of AS is needed to effectively rule out the disease.
To the best of our knowledge, an L4–5 nerve root radiculopathy associated with AS has not been previously reported. Increased suspicion may lead to earlier diagnosis and treatment, potentially reducing the duration of the symptoms and improving the functional ability of patient with AS.
Case Report
We present the case of a 37-year-old male patient with no significant PMH and a past surgical history of bilateral hip arthroplasty secondary to idiopathic aseptic necrosis of the bilateral femoral head and bilateral rotator cuff repaired surgery due to multiple motor vehicle accidents (MVA).
He came to our clinic for occasional low back pain in early 2018. Initially, he rated the pain as an 8 out of 10 on the numerical rating scale (NRS). It was described as a constant, aching sensation that radiated from his lower back to both feet with associated stiffness, numbness, and tingling in his feet. His pain was mostly concentrated in his lower back and gluteal regions. The intensity of his pain was more significant in the morning and when a posture was maintained for a prolonged duration.
On the initial visit, the physical examination elicited that the patient had limited thoracic and lumbar spinal range of motion throughout the sagittal and coronal planes, most apparent on the left side. As for the special test, the FABER test was positive on the left side, the straight leg raise was negative, and the Schober test was positive (Lumbar flexion difference is 4 cm). There were bilateral lumbar paraspinal spasms. Muscle strength, light touch, sensations, tactile discrimination, and deep tendon reflexes were normal in all extremities (Table 1). His complete blood count, complete metabolic panel, other antibody tests and inflammatory markers were within the reference ranges except for a mildly elevated total complement level and low hemoglobin level (Table 2).
Table 1.
Muscle strength grading and deep tendon reflexes (on last visit).
| MRC* | |||
|---|---|---|---|
| Upper limbs | Right | Left | |
| Wrist extension | 5/5 | 5/5 | |
| Wrist flexion | 5/5 | 5/5 | |
| Elbow extension | 5/5 | 5/5 | |
| Elbow flexion | 5/5 | 5/5 | |
| Shoulder abduction | 5/5 | 5/5 | |
| Lower limbs | Right | Left | |
| Ankle dorsiflexion | 5/5 | 5/5 | |
| Ankle plantarflexion | 5/5 | 5/5 | |
| Knee extension | 5/5 | 5/5 | |
| Knee flexion | 5/5 | 5/5 | |
| Hip flexion | 5/5 | 5/5 |
| DTR** | |||
|---|---|---|---|
| Lower limbs | Right | Left | |
| Patellar | 2/4 | 2/4 | |
| Ankle | 2/4 | 2/4 | |
| Upper limbs | Right | Left | |
| Brachioradialis | 2/4 | 2/4 | |
| Biceps | 2/4 | 2/4 | |
| Triceps | 2/4 | 2/4 | |
| Triceps | 2/4 | 2/4 | |
| Plantar response | Negative reflexes bilaterally | ||
| Faber test | Positive on the left side only | ||
| Straight leg raise | Negative bilaterally | ||
| Schober test | Positive (lumbar flexion difference is 4 cm) |
Medical Research Council muscle strength scale (Graded 0–5);
NINDS (National Institute of Neurological Disorders and Stroke) Myotatic Reflex Scale for deep tendon reflexes (Graded 0–4). MRC – Medical Research Council; DTR – deep tendon teflexes.
Table 2.
Patient’s laboratory tests summary (obtained on initial visit).
| Test | Result | Reference range |
|---|---|---|
| Completement, total | >60 | 31–60 U/mL |
| Completement component C3 | 156 | 82–185 mg/dL |
| Completement component C4 | 36 | 15–53 mg/dL |
| Rheumatoid factor | <14 | <14 IU/mL |
| C-reactive protein | 2.2 | <8.0 mg/L |
| Sjogren’s antibody (SS-A) | <1.0 NEG | <1.0 NEG |
| Sjogren’s antibody (SS-B) | <1.0 NEG | <1.0 NEG |
| Proteinase-3 antibody | <1.0 (no antibody detected | <1.0 |
| Myeloperoxidase antibody | <1.0 (no antibody detected) | <1.0 |
| HLA-B27 antigen | 6.9 (Negative) | 5.0–11.0 mcg/mL |
| ANA screen, IFA | Negative | Negative |
| HIV antigen/antibody, 4th generation | Non-reactive | Non-reactive |
| Hepatitis C antibody | Non-reactive | Non-reactive |
| Cyclic citrullinated peptide antibody | <16 (negative) | <20 |
| VDRL | Non-Reactive | Non-reactive |
| Red blood cell count | 4.18 | 4.20–5.80 Million/uL |
| Hemoglobin | 12.7 | 13.2–17.1 g/dL |
| Hematocrit | 35.4 | 38.5–50.0% |
| Glucose | 115 | 65–99 mg/dL |
SS-A – anti-Sjogren’s-syndrome-related antigen A autoantibodies; SSB – anti-Sjögren’s-syndrome-related antigen B autoantibodies; HLA – human leukocyte antigens; ANA – antinuclear antibodies; IFA – immunofluorescence assay; HIV – human immunodeficiency virus; VDRL – venereal disease research laboratory test.
On magnetic resonance imaging (MRI), there was evidence of an L4–5 mild broad-based disc bulge with superimposed small central disc protrusion/herniation and traced bilateral facet joint effusions along with sclerosis, joint space narrowing, and erosions of bilateral sacroiliac joints (Figures 1, 2). In addition, electromyography (EMG) was abnormal for chronic left L5 motor radiculopathy with signs of healing, but the nerve conduction studies were normal (Table 3). Therefore, a diagnosis of lumbar radiculopathy was corroborated based on clinical features, MRI findings, and EMG report.
Figure 1.
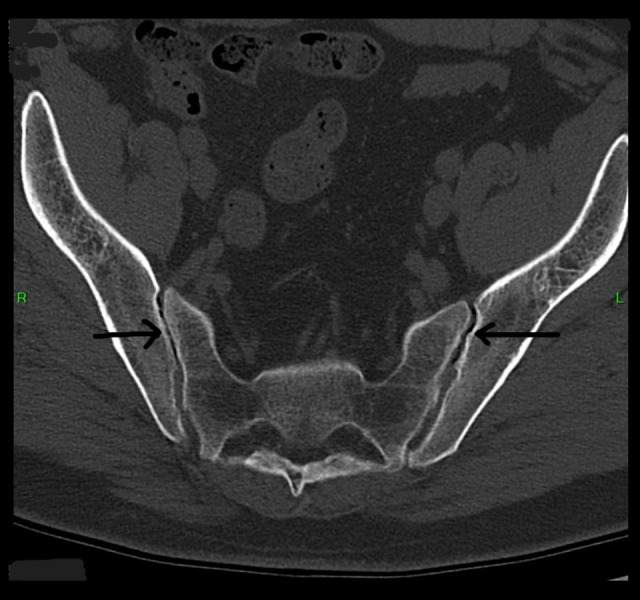
Axial view non-contrast CT scan of the pelvis showing bilateral Sacroiliitis and bony erosions (black arrows).
Figure 2.
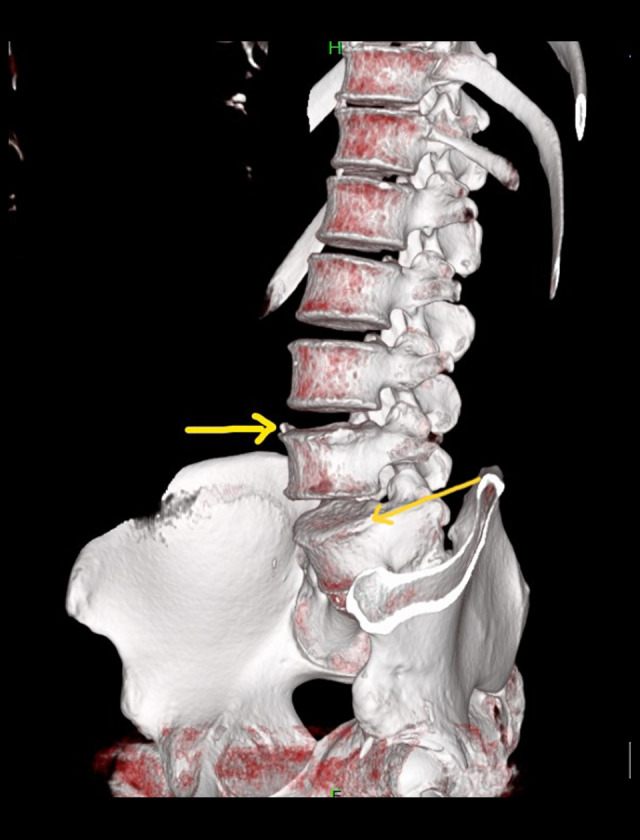
Sagittal view non-contrast CT scan of the lumbar spine using volume rendering technique (VRT) showing syndesmophytes over L4 and L5 vertebrae (yellow arrows).
Table 3.
Needle EMG (Electromyogram) results obtained on the follow-up visit.
| Side | Muscle | Nerve | Root | Ins Act | Fibs | Psw | Amp | Dur | Poly | Recrt | Int Pat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Vastus med | Femoral | L2–4 | Nml | Nml | Nml | Nml | Nml | 0 | Nml | Nml |
| Right | Ant tibialis | Dp Br fibular | L4–5 | Nml | Nml | Nml | Nml | Nml | 0 | Nml | Nml |
| Right | Fibularis long | Sup Br fibular | L5-S1 | Nml | Nml | Nml | Nml | Nml | 0 | Nml | Nml |
| Right | Gastroc | Tibial | S1–2 | Nml | Nml | Nml | Nml | Nml | 0 | Nml | Nml |
| Left | Vastus med | Femoral | L2–4 | Nml | Nml | Nml | Nml | Nml | 0 | Nml | Nml |
| Left | Ant tibialis | Dp Br fibular | L4–5 | Nml | Nml | Nml | Nml | Nml | 1+ | Nml | Nml |
| Left | Fibularis long | Sup Br fibular | L5-S1 | Nml | Nml | Nml | Nml | Nml | 1+ | Nml | Nml |
| Left | Gastroc | Tibial | S1–2 | Nml | Nml | Nml | Nml | Nml | 0 | Nml | Nml |
Needle EMG study of bilateral lower extremity shows normal pattern except for left anterior tibialis and left fibularis longus which had increased polyphasic (L5 nerve root). Nml – normal; Vastus med – vastus medialis muscle; Ant tibialis – anterior tibialis muscle; Fibularis long – fibularis longus muscle; Gastroc – gastrocnemius muscle; Ins act – insertional activity; Fibs – fibrillation; Psw – positive sharp wave; Amp – amplitude; Dur – duration; Poly – polyphase; Recrt – recruitment; Int pat – interval pattern; Dp Br fibular – deep branch of fibular nerve; Sup Br fibular – superficial branch of fibular nerve.
Over the course of three and a half years, the patient had inadequate pain relief with multiple NSAIDs, pain medications (Oxycontin and Percocet), tizanidine, multiple sessions of osteopathic manipulative treatment (OMT), Physical therapy (PT), and multiple trigger point injections. He also underwent one left Intralaminar lumbar epidural steroid injection (L4/5) with 1-month relief of low back pain and resolution of radiating left leg pain and three bilateral SI joint injections, which provided >50% improvement in his low back pain for 2–3 weeks. In Feb 2021, while the patient was waiting for the insurance approval for another intralaminar L4–5 lumbar epidural steroid injection, he developed bilateral eye redness and pain. At this point, point, we considered his clinical symptoms to be an unusual presentation of AS possibly, and he was referred to an ophthalmologist and a rheumatologist, where he was diagnosed with AS. Adalimumab was initiated, and the patient experienced gradual improvement in his low back pain, radicular lower legs pain, and bilateral SI joints pain, with reduced need of pain medications. In a follow-up visit one month after initiating adalimumab, he rated his lower back and bilateral SI joint pain as 3–4/10 on the NRS. As a result, he could return to work and resume many of his activities.
Discussion
We present a rare case of lumbar radiculopathy associated with ankylosing spondylitis that was reversed with adalimumab. Despite conventional pain management, he experienced 3 years of intractable lower back pain with paresthesia throughout both lower limbs. After a diagnosis was corroborated using EMG, MRI, and clinical assessment, the patient was prescribed adalimumab and had relief of his symptoms within one month. To the best of our knowledge, attenuation of radicular pain with adalimumab in a case of ankylosing spondylitis has not been previously reported. Like our patient’s presentation, Forestier et al stated that pain resembling sciatica is present in the early stage of the disease in 17 out of 200 patients. Most of these cases were described as having radiating pain in the distribution of the L5 nerve root [13,14]. Interestingly, L5 motor radiculopathy was characterized in the present case of AS.
The spine is progressively involved as the disease progresses, and previous studies using SSEP (somatosensory evoked potential) depict a prevalence of lumbosacral radiculopathy in AS to be 6.7–16.7%, although most cases were subclinical [15]. However, the neurological manifestations of AS are quite variable, ranging from minor joint instabilities to cauda equina syndrome, and they are rarely reported [16–18]. Bowie and Glasgo first described cauda equina syndrome as the rare complication of AS involving the dorsal and ventral roots of L3–S5 [19]. Similarly, Cumming and colleagues reported an association between AS and upper limb radiculopathy in addition to cauda equina syndrome during the late stages of the disease [20]. Those reports bear a resemblance to our patient’s presentation, but our patient’s neurological symptoms occurred at the early stage of the disease.
Several mechanisms have been postulated as causes of nervous system involvement in AS, such as arteritis, demyelination, cord, and nerve root compression from inflammation [16,21–24]. The intervertebral foramina may be radiologically normal, but the root pain is attributed to inflammatory changes and the development of characteristic bony spurs: syndesmophytes as in our patient’s CT scan [25–28]. Additionally, the immune-mediated inflammatory responses in AS may contribute to enthesis and the erosive changes at the junction of the vertebrae and the annulus fibrosis. As a result, the extensive remodeling of the spine and spinal stenosis ensues due to bone overgrowth [16,25,28]. Given his diagnosis of AS with radicular symptoms, we presume that these immune-mediated mechanisms played a role in the present case.
On the other hand, lumbosacral radiculopathy is one of the most common symptoms presented to the outpatient clinic and is a leading referral diagnosis of EMG. The prevalence of lumbosacral radiculopathy is 3–5%, equally distributed between men and women in the general population [27–29]. Additionally, the back pain from lumbar radiculopathy and the inflammatory back pain from spondyloarthropathy have similar presentations in the early stage of AS [13,30]. Despite his relatively young age, it is plausible that his lower back pain was due to a history of multiple motor vehicle accidents. However, with a history of being recalcitrant to the opioid medications, multiple trigger point injections, epidural steroid injections, and osteopathic manipulative treatment for 3 years and his subsequent pain relief from initiation of adalimumab suggest that the probable cause of radiculopathy was AS.
Moreover, the criteria used for diagnosing AS, such as the modified New York criteria, require radiographic evidence of sacroiliitis [31]. This usually challenges the physicians and hampers the diagnosis because it takes years from the onset of the symptoms to the appearance of radiographic sacroiliitis. Sacroiliac joint inflammation occurs prior to the state that is detectable radiographically [31,32]. Many unnecessary investigations and treatments may have been performed during this prolonged diagnosis delay.
The diagnosis of AS is primarily clinical, and there are no specific laboratory investigations that can confirm the disease. HLA is strongly associated with the disease, and >80% of patients with AS are positive for HLA B27. Routine HLA testing is not clinically helpful because AS can occur in the absence of the HLA B27, as in the present case [2,33,34]. Further, only 1–5% of HLA-B27-positive individuals develop AS. Therefore, it is likely that there exists a combination of mechanisms involved in the disease pathogenesis [35].
NSAIDs (non-steroidal anti-inflammatory drugs) are the first-line recommended treatment for active AS [36]. Theoretically, NSAIDs inhibit the activity of COX (cyclooxygenase) enzymes, thereby inhibiting the synthesis of prostaglandins (PG) and thromboxane. PGE2 acts through PTGER4 (Prostaglandin E Receptor 4) to induce the production of IL (Interleukin)-23 and IL-17 and to promote the expansion of TH17 lymphocyte. Elevated TH17 lymphocyte counts and IL-17 levels are very well known in AS [35–37]. Reportedly, AS is highly associated with genetic variation in the PTGER4 gene, which leads to resistance to NSAIDs, like in our patient’s poor response to multiple NSAIDs [38]. European League Against Rheumatism (EULAR) guidelines recommend that biologic disease-modifying antirheumatic drugs (eg, Tumor necrosis factor inhibitors and IL-17 inhibitors) should be considered in patients with persistently high disease activity despite conventional treatments with NSAIDs [38].
Timely initiation of the tumor necrosis factor (TNF) blocking agent is necessary for managing spondyloarthropathies because it can dramatically decrease disease activity, improving the patient’s symptoms and radiographic sacroiliitis [36–39]. In our case report, use of adalimumab (TNF blocking agent) alleviated the recalcitrant low back pain with radicular symptoms and reduced the need for opioid medications.
Limitations of the Study
We aimed to present the unusual presentation of AS with lumbar radiculopathy signs and symptoms. However, there are limitations of our study: (1) the patient had previous multiple accidents that could contribute to the lumbar disc bulging, and (2) he should have had follow-up blood work to monitor complement level and MRI imaging to identify the improvement of radiographic sacroiliitis after initiation of adalimumab.
Conclusions
Current evidence demonstrates the beneficial effects of TNF inhibitor (adalimumab) in AS patient with symptoms of radiculopathy. It provides sustained clinical remission with the restoration of normal physical activities. Young patients with chronic worsening low back pain with symptoms of radiculopathy, reduced spine mobility, and minimal relief from opioid medications and epidural steroid injection should be referred to a rheumatologist to rule out AS. HLA-B27 is not a diagnostic feature for AS, and diagnostic delay can lead to unnecessary treatment, with poor quality of life, worse functional impairment, and more significant radiographic progression [40]. As such, physicians should be aware of the features of inflammatory low back pain.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Stolwijk C, Boonen A, van Tubergen A, Reveille JD. Epidemiology of spondyloarthritis. Rheum Dis Clin North Am. 2012;38(3):441. doi: 10.1016/j.rdc.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkoç N, Yarkan H, Kenar G, Khan MA. Ankylosing spondylitis: HLAB*27-positive versus HLA-B*27-negative disease. Curr Rheumatol Rep. 2017;19(5):26. doi: 10.1007/s11926-017-0654-8. [DOI] [PubMed] [Google Scholar]
- 3.Poddubnyy D, Rudwaleit M. Early spondyloarthritis. Rheum Dis Clin North Am. 2012;38(2):387–403. doi: 10.1016/j.rdc.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Østergaard M, Lambert RGW. Imaging in ankylosing spondylitis. Ther Adv Musculoskelet Dis. 2012;4(4):301–11. doi: 10.1177/1759720X11436240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/ EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011;70(6):896–904. doi: 10.1136/ard.2011.151027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Heijde D, Sieper J, Maksymowych WP, et al. 2010 Update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis. 2011;70(6):905–8. doi: 10.1136/ard.2011.151563. [DOI] [PubMed] [Google Scholar]
- 7.Vastesaeger N, van der Heijde D, Inman RD, et al. Predicting the outcome of ankylosing spondylitis therapy. Ann Rheum Dis. 2011;70(6):973–81. doi: 10.1136/ard.2010.147744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.S van der L, D van der H. Ankylosing spondylitis. Clinical features. Rheum Dis Clin North Am. 1998;24(4):48–52. doi: 10.1016/s0889-857x(05)70036-3. [DOI] [PubMed] [Google Scholar]
- 9.Lukas C, Cyteval C, Dougados M, Weber U. MRI for diagnosis of axial spondyloarthritis: Major advance with critical limitations “Not everything that glisters is gold (standard).”. Open. 2018;4:586. doi: 10.1136/rmdopen-2017-000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin A, Taurog JD. The spondylarthritides. Published online 1998;353.
- 11.Moll JMH, Wright V. New York clinical criteria for ankylosing spondylitis. A statistical evaluation. Ann Rheum Dis. 1973;32(4):354. doi: 10.1136/ard.32.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34(11):1074–77. doi: 10.1093/rheumatology/34.11.1074. [DOI] [PubMed] [Google Scholar]
- 13.O’Shea F, Salonen D, Inman R. The challenge of early diagnosis in ankylosing spondylitis. J Rheumatol. 2007;34(1):5–7. [PubMed] [Google Scholar]
- 14.Forestier J, Jacqueline F. Rotes-Querol. Ankylosing spondylitis. Published online 1956. [Google Scholar]
- 15.Matthews WB. The neurological complications of ankylosing spondylitis. J Neurol Sci. 1968;6(3):561–73. doi: 10.1016/0022-510x(68)90035-x. [DOI] [PubMed] [Google Scholar]
- 16.Khedr EM, Rashad SM, Hamed SA, et al. Neurological complications of ankylosing spondylitis: Neurophysiological assessment. Rheumatol Int. 2009;29(9):1031–40. doi: 10.1007/s00296-009-0841-7. [DOI] [PubMed] [Google Scholar]
- 17.Carman RD, And Fineman S, Harrington SW, et al. Ankylosing spondylitis: (An analysis of 100 cases) CMAJ. 1947;57(1):16. [PubMed] [Google Scholar]
- 18.Kotil K, Yavasca P. Lumbar radiculopathy in ankylosing spondylitis with dural ectasia. J Clin Neurosci. 2007;14(10):981–83. doi: 10.1016/j.jocn.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Bowie EA, Glasgow GL. Cauda equina lesions associated with ankylosing spondylitis. Br Med J. 1961;2:24–27. doi: 10.1136/bmj.2.5243.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cumming WJK, Saunders M. Radiculopathy as a complication of ankylosing spondylitis. J Neurol Neurosurg Psychiatry. 1978;41(6):569–70. doi: 10.1136/jnnp.41.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos-Remus C, Gomez-Vargas A, Guzman-Guzman JL, et al. Frequency of atlantoaxial subluxation and neurologic involvement in patients with ankylosing spondylitis. J Rheumatol. 1995;22(11):2120–25. [PubMed] [Google Scholar]
- 22.Lan HHC, Chen DY, Chen CCC, et al. Combination of transverse myelitis and arachnoiditis in cauda equina syndrome of long-standing ankylosing spondylitis: MRI features and its role in clinical management. Clin Rheumatol. 2007;26(11):1963–67. doi: 10.1007/s10067-007-0593-2. [DOI] [PubMed] [Google Scholar]
- 23.Tyrrell PNM, Davies AM, Evans N. Neurological disturbances in ankylosing spondylitis. Ann Rheum Dis. 1994;53(11):714–17. doi: 10.1136/ard.53.11.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JY, Kim J il, Park JY, et al. Cervical spine involvement on longstanding ankylosing spondylitis. Clin Exp Rheumatol. 2005;23(3):331–39. [PubMed] [Google Scholar]
- 25.Ramos-Remus C, Russell AS, Gomez-Vargas A, et al. Ossification of the posterior longitudinal ligament in three geographically and genetically different populations of ankylosing spondylitis and other spondyloarthropathies. Ann Rheum Dis. 1998;57(7):429–33. doi: 10.1136/ard.57.7.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi M, Kawanami H, Tomonaga M, Kitamura K. Ossification of the posterior longitudinal ligament – a roentgenologic and clinical investigation. Acta Radiol Diagn (Stockh) 1972;13(1):25–36. doi: 10.1177/02841851720130p105. [DOI] [PubMed] [Google Scholar]
- 27.Tarulli AW, Raynor EM. Lumbosacral radiculopathy. Neurol Clin. 2007;25(2):387–405. doi: 10.1016/j.ncl.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Siré E, Van Der Heijde D, Rgen Braun J, Deodhar A, et al. Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology (Oxford) 2019;58(3):388–400. doi: 10.1093/rheumatology/key128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedly J, Standaert C, Chan L. Epidemiology of spine care: The back pain dilemma. Phys Med Rehabil Clin N Am. 2010;21(4):659–77. doi: 10.1016/j.pmr.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–68. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 31.Mau W, Zeidler H, Mau R, et al. Clinical features and prognosis of patients with possible ankylosing spondylitis. Results of a 10-year followup. J Rheumatol. 1988;15(7):1109–14. [PubMed] [Google Scholar]
- 32.Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: Do we need new criteria? Arthritis Rheum. 2005;52(4):1000–8. doi: 10.1002/art.20990. [DOI] [PubMed] [Google Scholar]
- 33.Caffrey MFP, James DCO. Human lymphocyte antigen association in anky-losing spondylitis. Nature. 1973;242(5393):121. doi: 10.1038/242121a0. [DOI] [PubMed] [Google Scholar]
- 34.Brewerton DA, Hart FD, Nicholls A, et al. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1(7809):904–7. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 35.Evans DM, Spencer CCA, Pointon JJ, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011;43(8):761–67. doi: 10.1038/ng.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blair HA. Secukinumab: A review in ankylosing spondylitis. Drugs. 2019;79:433–43. doi: 10.1007/s40265-019-01075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60(6):1647–56. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 38.de Koning A, Schoones JW, van der Heijde D, et al. Pathophysiology of axial spondyloarthritis: Consensus and controversies. Eur J Clin Invest. 2018;48(5):e12913. doi: 10.1111/eci.12913. [DOI] [PubMed] [Google Scholar]
- 39.Zhu W, He X, Cheng K, et al. Ankylosing spondylitis: Etiology, pathogenesis, and treatments. Bone Res. 2019;7:22. doi: 10.1038/s41413-019-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao SS, Pittam B, Harrison NL, et al. Diagnostic delay in axial spondylo-arthritis: A systematic review and meta-analysis. Rheumatology (Oxford) 2021;60(4):1620–28. doi: 10.1093/rheumatology/keaa807. [DOI] [PubMed] [Google Scholar]


