Figure 2: Potential Guideline-directed Medical Therapy Optimisation Strategies.
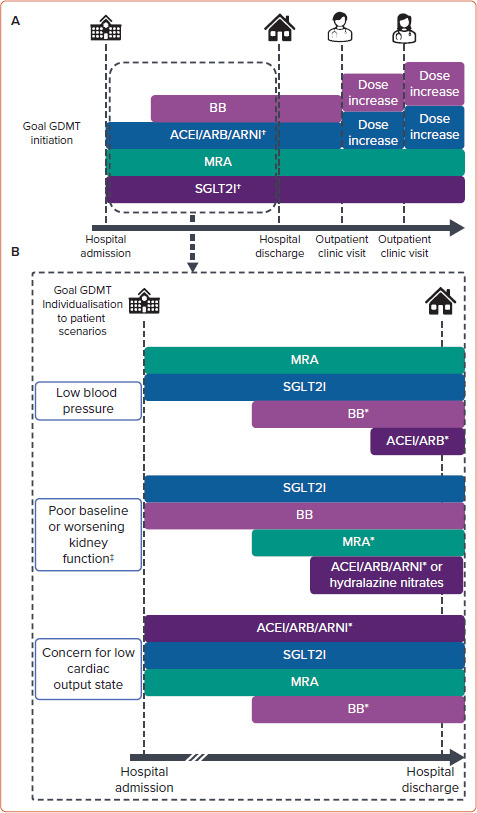
A: Goal initiation and titration timeline of GDMT continuation and initiation during and following an acute decompensated heart failure hospitalisation in a patient with no contraindications to therapies presenting with a ‘warm and wet’ haemodynamic profile. B: Example of how this goal in-hospital timeline can be modified for patient scenarios with barriers to the proposed in-hospital timeline in (A). More time may be needed between hospital admission and medication initiation or titration in patients with initial barriers to GDMT initiation or titration (indicated by dashed timeline). SGLT2Is have evidence for initiation with an estimated glomerular filtration rate (eGFR) of 20 ml/min/m2 or more. MRAs have evidence for initiation with an eGFR of 30 ml/min/m2 or more or may require potassium binders to use at lower eGFR. *Medication can be initiated once clinical status (i.e. blood pressure, kidney function, haemodynamic assessment) is appropriate. †Clinical trial evidence to support in-patient initiation. ‡Mild–moderate worsening kidney function with clinical status improvement during decongestion with diuresis should not alter GDMT initiation or titration goals unless the magnitude of worsening kidney function is unacceptable. ACEI = angiotensinconverting enzyme inhibitor; ARB = angiotensin-receptor blocker; ARNI = angiotensin receptor–neprilysin inhibitor; BB = β-blocker; GDMT = guideline-directed medical therapy; MRA = mineralocorticoid receptor antagonist; SGLT2Is = sodium–glucose cotransporter 2 inhibitors.
