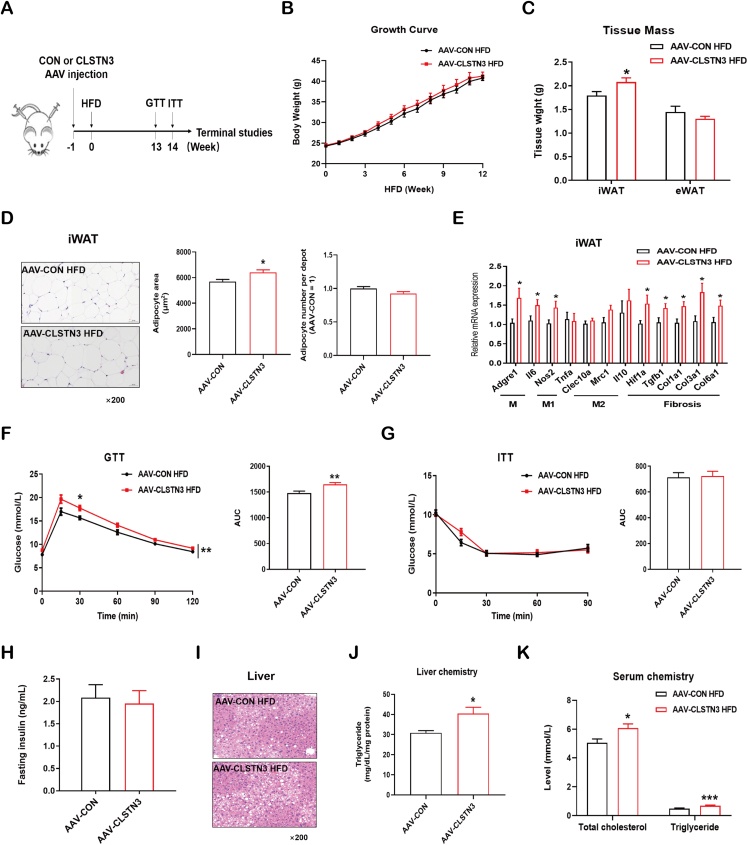
Figure 4.
CLSTN3 overexpression deteriorates diet-induced iWAT dysfunction and liver steatosis. (A) Schematic representation of AAV injection in iWAT, and experimental procedures for AAV-CON and AAV-CLSTN3 mice fed a high-fat diet (HFD) for 12–15 weeks. (B) Growth curve of body weight during 12-week HFD (n = 8–9). (C) Tissue mass of iWAT and epididymal white adipose tissue (eWAT) at 15-week HFD (n = 7–9). (D) Representative hematoxylin and eosin (H&E) staining images of iWAT sections at 15-week HFD, and its quantification of adipocyte size and number. Scale bar, 50 μm. (E) Quantitative PCR analysis of mRNA expression of inflammation (generic macrophage signature gene: Adgre1 and Il6; M1-like: Nos2 and Tnfa; M2-like: Clec10a, Mrc1, and Il10) and fibrosis (Hif1a, Tgfb1, Col1a1, Col3a1, and Col6a1) related genes in iWAT from AAV-CON and AAV-CLSTN3 mice upon 15-week HFD (n = 9). (F) Glucose tolerance test (GTT) performed in AAV-CON and AAV-CLSTN3 mice after 13-week HFD, and its area under the curve (AUC) (n = 7–8). (G) Insulin tolerance test (ITT) performed upon 14-week HFD, and its AUC (n = 6–9). (H) Fasting insulin concentration measured in serum at 15-week HFD (n = 8–9). (I) Representative H&E staining images of liver sections at 15 weeks of HFD. Scale bar, 50 μm. (J) Triglyceride levels in liver from AAV-CON and AAV-CLSTN3 groups (n = 9–10). (K) Total cholesterol and triglyceride levels in serum from AAV-CON and AAV-CLSTN3 mice fed with 15-week HFD (n = 9–10). Data are shown as mean ± SEM of biologically independent samples, and ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.