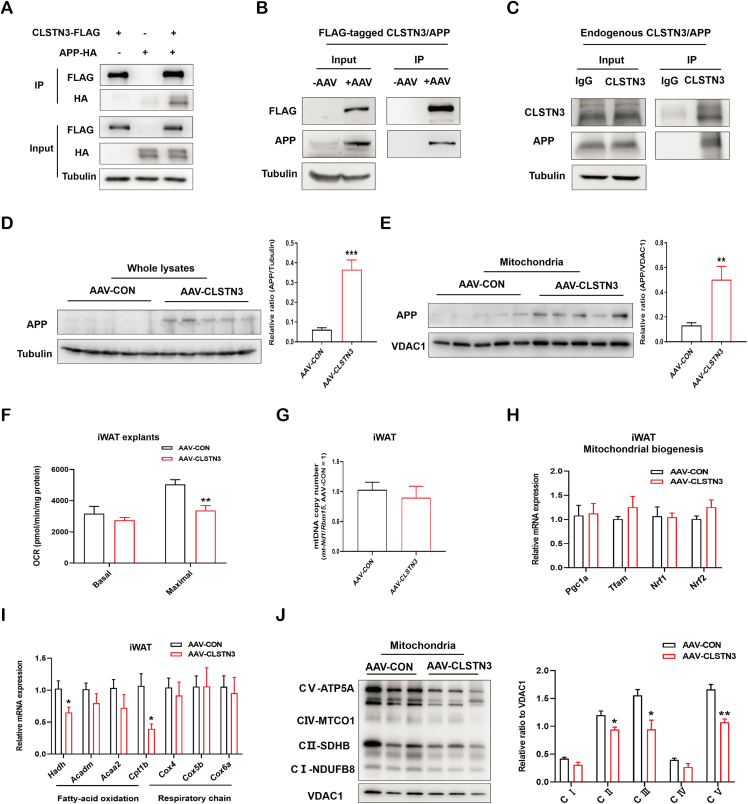
Figure 6.
CLSTN3 associates with APP and represses adipose mitochondrial function. (A) Immunoprecipitation of CLSTN3 with APP in 293T cells co-transfected with CLSTN3-FLAG and APP-HA plasmids for 48 h. Cell lysate (input) and pull-down (IP) of FLAG-tagged CLSTN3 were immunoblotted with indicated antibodies. (B) Immunoprecipitation of FLAG-tagged CLSTN3 and APP in iWAT from AAV-CON and AAV-CLSTN3 mice. (C) Immunoprecipitation of endogenous CLSTN3 and APP in human SAT. (D and E) Immunoblot analysis and the quantification for APP levels in whole-tissue lysate (D) and mitochondria (E) isolated from iWAT in AAV-CON and AAV-CLSTN3 mice; VDAC1, mitochondrial protein marker. (F) Ex vivo mitochondrial respiration in iWAT explants from AAV-CON and AAV-CLSTN3 mice; basal and maximal oxygen consumption rate (OCR) normalized to the protein content were presented (n = 5). (G) Mitochondrial DNA (mtDNA) copy number in iWAT from two groups (n = 5). (H) Quantitative PCR analysis of genes involved in mitochondrial biogenesis (Pgc1a, Tfam, Nrf1, and Nrf2) in iWAT from AAV-CON and AAV-CLSTN3 mice (n = 5). (I) Quantitative expression of genes involved in fatty-acid oxidation (Hadh, Acadm, Acaa2, and Cpt1b) and respiratory chain (Cox4, Cox5b, and Cox6a) in iWAT from AAV-CON and AAV-CLSTN3 mice (n = 5). (J) Immunoblot analysis and the quantification for crucial OXPHOS components, including NDUFB8 (CⅠ), SDHB (CII), UQCRC2 (CIII), MTCO1 (CⅣ), and ATP5A (CV), in mitochondria isolated from iWAT in AAV-CON and AAV-CLSTN3 mice. Data are shown as mean ± SEM of biologically independent samples, and ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.