Figure 2.
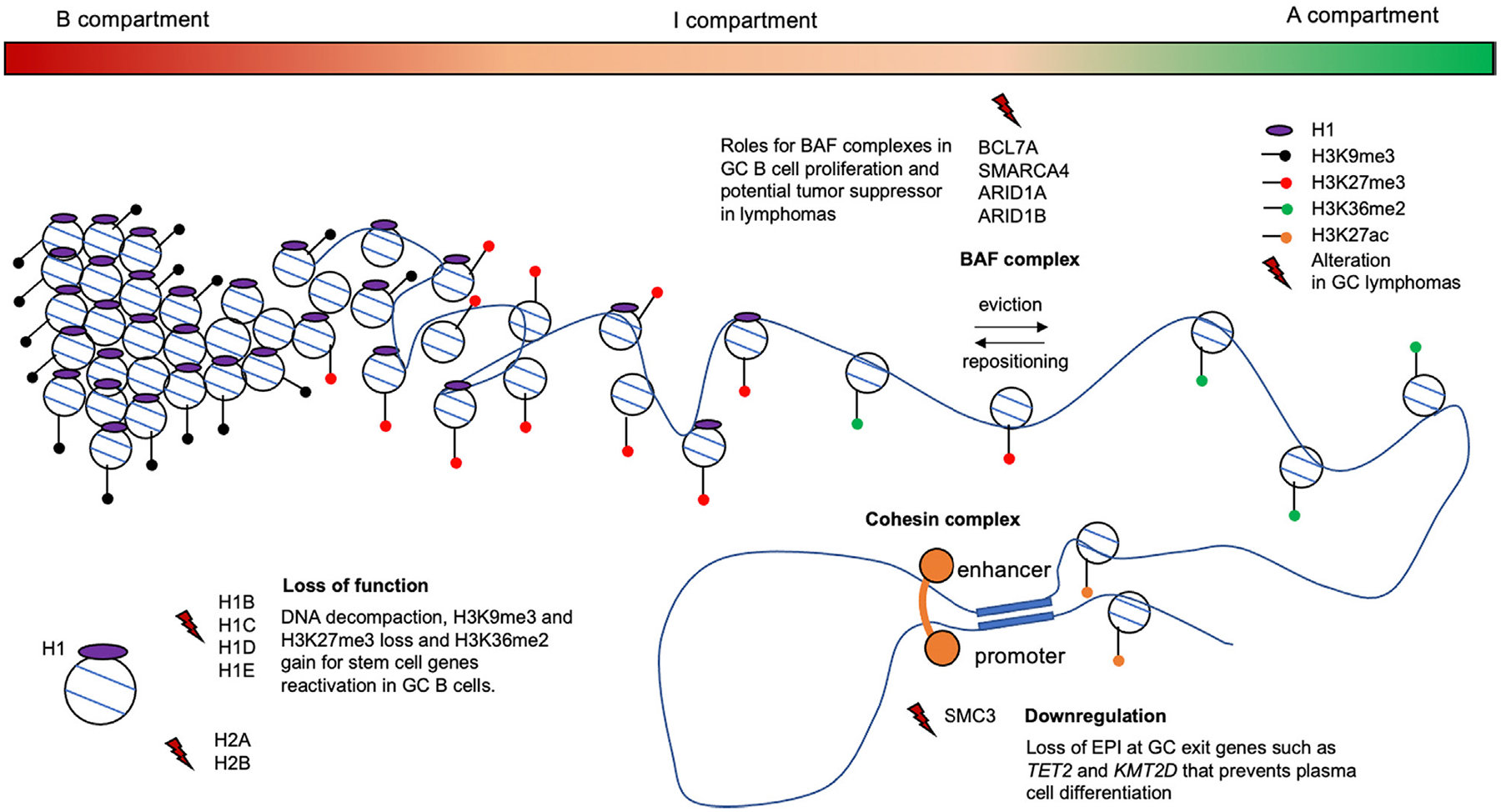
Graphical representation of chromatin architecture regulatory protein alterations in GC-derived lymphomas. The genome ranges from highly compacted to decompacted through three continuous compartments (B to I to A). These are associated with histone marks that either silence (H3K9me3 and H3K27me3) or facilitate activation (H3K36me2) of gene expression. The gradient of H1 density is associated with the range of chromatin compaction and hence epigenetic and transcriptional activation potential. H1 loss of function mutations lead to decompaction and activation of embryonic stem cell genes. Cohesin complexes create chromatin loops such as EPIs. SMC3 haploinsufficiency impairs GC B-cell differentiation by reducing such EPIs at GC exit genes and tumor suppressors thus contributing to lymphomagenesis. BAF subunits are involved in nucleosome ejection and repositioning and are frequently mutated in GC lymphomas. Their precise role in lymphomagenesis still needs to be clarified, but recent studies suggest that BAF components have a tumor suppressor role [30]. Histone H1 proteins are represented in purple, H3 post-modifications by dots: H3K9me3 (black), H3K27me3 (red), H3K36me2 (green), H3K27ac (orange) and lightning indicate alterations of chromatin regulatory proteins and histones.
