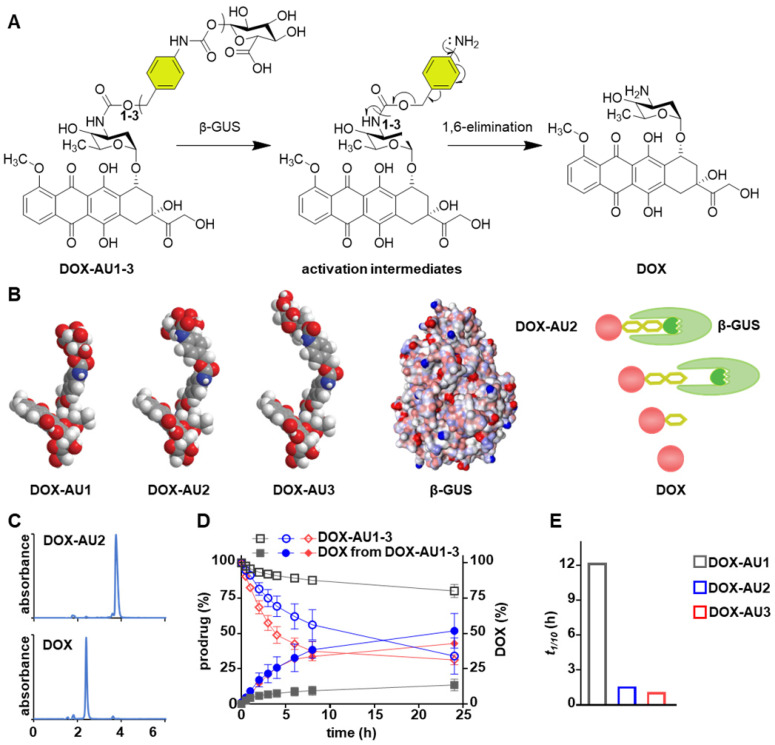
Figure 2.
Enzymatic activation of glucuronide-capped self-immolative doxorubicin prodrugs. (A) β-GUS-mediated conversion of DOX-AU1-3 to the parent drug DOX. β-GUS first digests the glucuronide moiety in the prodrugs, followed by self-immolation of the spacer via elimination of the aromatic units. (B) Increasing the spacer length by adding more aromatic units reduces steric hindrance. It facilitates the insertion of the glucuronide moiety into the catalytic pocket of β-GUS, resulting in faster and more efficient prodrug activation. (C) HPLC chromatograms of DOX-AU2 and DOX after enzymatic activation. (D) Kinetics of DOX-AU1-3 degradation and DOX generation upon exposure to β-GUS at pH 7.4 and 37 °C. Enzymatic activation was significantly faster for prodrugs with spacers containing more than one aromatic unit. (E) The time needed to generate 10% (t1/10) of DOX upon enzyme exposure was significantly shorter for prodrugs with spacers containing more than one aromatic unit. Due to the very slow activation of DOX-AU1, t1/10 was analyzed instead of t1/2.