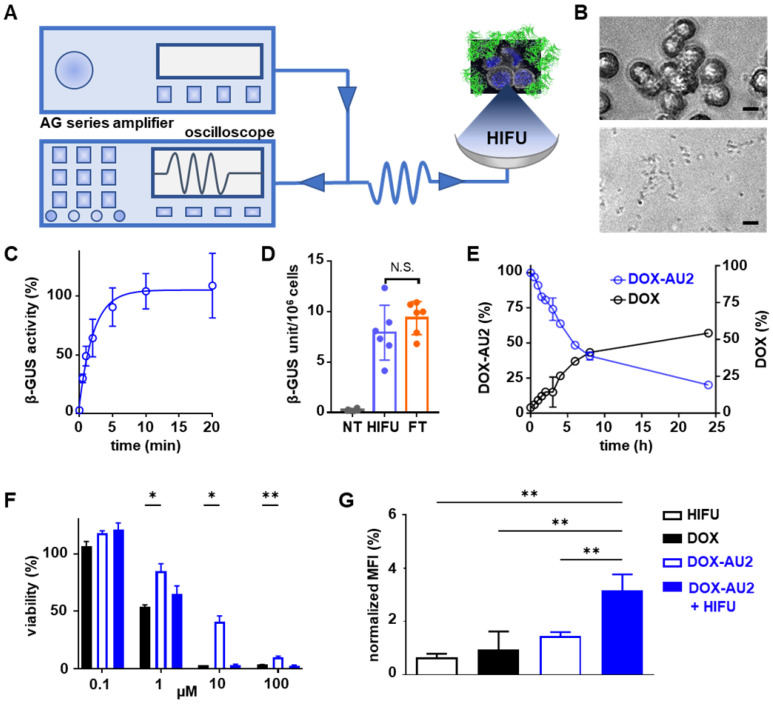
Figure 4.
Focused ultrasound-induced mechanical cell destruction induces β-GUS release and promotes prodrug activation. (A) Schematic HIFU setup, composed of an amplifier/generator and an oscilloscope. The HIFU transducer spatially focuses the US energy and mechanically destroys tumor cells to release intracellular β-GUS into the environment for prodrug activation. (B) Bright-field microscopy images of 4T1 breast cancer before (upper) and after (lower) HIFU treatment for 10 min at 41 MPa peak-negative pressure (scale bar 20 µM). (C) Release kinetics of β-GUS into the extracellular environment upon HIFU treatment. (D) Bioactivity of β-GUS released from HIFU-treated 4T1 cells as assessed by the MUG assay. Non-treated (NT) cells display very low extracellular β-GUS activity. Three cycles of freeze-thawing (FT) served as a positive control for cell lysis and β-GUS release. (E) DOX-AU2 conversion and DOX generation by β-GUS released from HIFU-treated 4T1 cells. (F) Cytotoxicity of DOX-AU2 in 4T1 cells in the presence and absence of β-GUS released from HIFU-treated 4T1 cells, showing that prodrug incubated with supernatant from HIFU-damaged cells exhibited similar cytotoxicity as parent DOX. (G) Flow cytometry analysis of calreticulin translocation in HIFU, DOX, DOX AU-2 and DOX AU-2 plus HIFU-lysate -treated 4T1 cells, showing significant amounts of calreticulin translocated to the outer surface of the cell membrane for prodrug plus HIFU-lysate, indicating induction of immunogenic cell death. Statistical differences were determined using two-way ANOVA with multiple comparison (F) and unpaired t-test (D, H). N.S. (non-significant), *p ≤ 0.05, **p ≤ 0.01.