Figure 8.
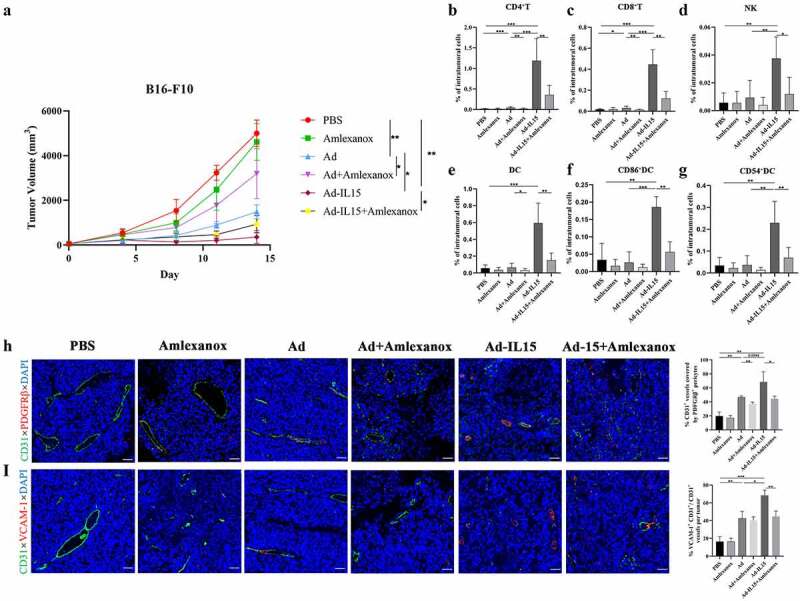
Amlexanox inhibits Ad-IL15-induced immune activation, tumor VN and nonclassical TLS formation. Mice bearing subcutaneous B16-F10 tumors (approximately 50 mm3) were intratumorally injected every other day for a total of three times with PBS, Amlexanox, Ad, Ad + Amlexanox, Ad-IL15, or Ad-IL15 + Amlexanox. (a)Tumor growth in mice treated with PBS, Amlexanox, Ad, Ad + Amlexanox, Ad-IL15, or Ad-IL15 + Amlexanox is shown. n=6–9. * P < .05, **P < .01 by Mann-Whitney U test. (b - g) Eleven days after the last treatment, tumors were collected and the percentage of immune cells was analyzed by flow cytometry. (b - d) Changes in the proportion of CD8+T cells, CD4+T cells and NK cells in the TME. (e - g) Changes in the proportion of DC, CD86+ DC and CD54+ DC in the TME. n = 6-9. * P < .05, **P < .01 and ***P ≤ .001 by Mann-Whitney U test. (h) Representative immunofluorescence staining and quantification of PDGFRβ+ pericyte coverage on tumor VECs. (i) Representative immunofluorescence staining and image quantification of VCAM-1 expression on tumor VECs. Statistical significance was assessed by Unpaired Student’s t test. *P ≤ .05, **P ≤ .01, ****P ≤ .0001. Scale bars: 50 μm.
