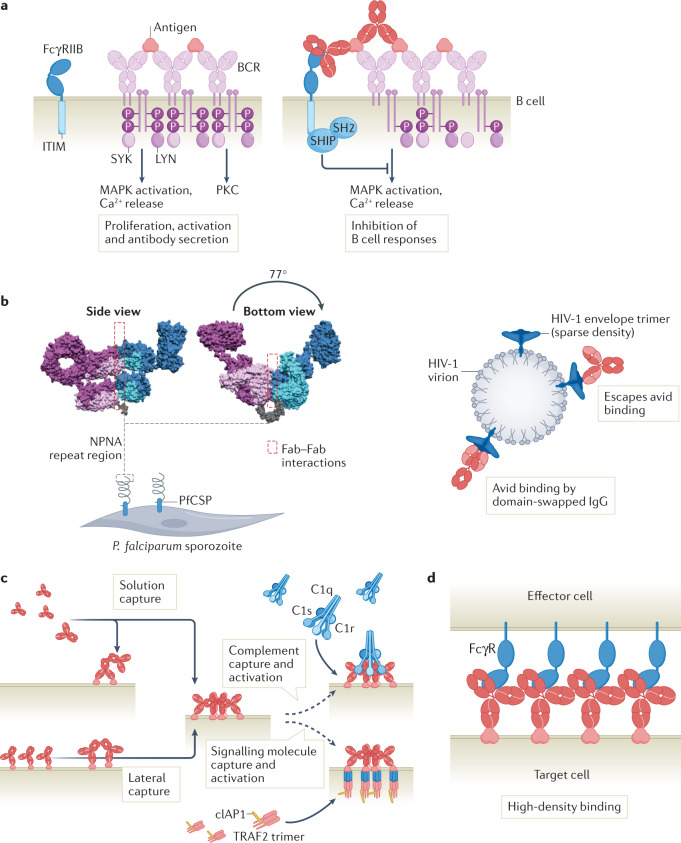
Fig. 3. Avidity in antibody biology.
a | B cell receptors (BCRs) constitute antibodies expressed with a transmembrane region on the B cell surface. First-order avidity interactions induce BCR crosslinking and phosphorylation of the BCR-associated transducer molecules that recruit SYK and LYN tyrosine kinases, resulting in protein kinase C (PKC) activation, mitogen-activated protein kinase (MAPK) activation and calcium release. Second-order and third-order avidity binding of antibody-antigen immune complexes recruit FcγRIIB, which comprises an immunoreceptor tyrosine-based inhibitory motif (ITIM motif) that activates phosphatases and reduces BCR signalling. b | Fab-domain-mediated neutralization of pathogen structures, preventing interactions with host cells and blocking pathogen entry into the cell. Binding of a protective antibody against the repetitive P. falciparum circumsporozoite protein (PfCSP) epitope consisting of repeats of the amino acids NPNA is facilitated by second-order avidity Fab–Fab interactions between Fabs from neighbouring IgGs (bound in pairs). Neighbouring Fabs each bind a two NPNA repeat and are shifted 77° along the NPNA spiral, with five IgGs completing a full circle. The Fabs of the IgGs are oriented non-symmetrically in a repeating light chain-bottom/heavy chain-top orientation. facilitating Fab–Fab contacts (left). HIV-1 virions escape second-order avidity binding by sparse surface expression of HIV-1 envelope glycoprotein spikes. The neutralizing antibody 2G12 evolved a domain-swapped structure in which each heavy chain contacts both light chains, thereby creating a large rigid surface that facilitates avid binding of conserved carbohydrate clusters on the HIV-1 envelope spike (right). c | Clustering of immune complexes is initiated by IgG molecules that assemble into ordered hexameric structures through non-covalent Fc–Fc interactions, which facilitate third-order avidity binding and activation of C1 or intracellular signalling through recruitment of signalling molecules such as cIAP1 and TRAF2. IgG hexamer formation proceeds through recruitment of additional IgG molecules through second-order avidity interactions mediated by the Fc domains until ring closure. d | Antibodies that predominantly bind monovalently (such as those with moderate affinity or those engineered for monovalent binding) may elicit stronger effector functions because higher cell surface densities of Fc domains can be achieved. Part a adapted from ref.41, Springer Nature Limited.