Abstract
Introduction
The oncology clinical trial recruitment process is time, labor, and resource intensive, and poor accrual rates are common. We describe the VA Connecticut Cancer Center experience of implementing a standardized, universal prescreening protocol and its impact on thoracic oncology research recruitment.
Methods
Research coordinators prescreened potentially eligible patients with confirmed or suspected cancer from multiple clinical sources and entered relevant patient and research study information into a centralized electronic database. The database provided real-time lists of potential studies for each patient. This enabled the research team to alert the patient's oncologist in advance of clinic visits and to prepare documents needed for enrollment. Clinicians could ensure sufficient time and attention in clinic to the informed consent process, therefore maximizing enrollment opportunities. Patients were also monitored on waitlists for future studies.
Results
From March 2017 to December 2020, a total of 1518 patients with lung nodules and suspected or confirmed lung cancers were prescreened. Of these, 379 patients were enrolled to a study, 103 patients declined participation, and 639 were monitored for future studies. Our prescreening protocol identified all new patients with lung cancer who were ultimately added to the cancer registry. We found a substantial increase in study enrollment after prescreening implementation.
Conclusions
Universal prescreening was associated with improved patient enrollment to thoracic oncology studies. The protocol was integral in our VA becoming the top accruing VA site for National Cancer Institute’s National Clinical Trials Network studies for 2019 to 2021.
Keywords: Clinical trial, Lung cancer, Accrual, Recruitment
Introduction
Therapeutic developments and insights gained through clinical research are essential to thoracic oncology. Nevertheless, the clinical research recruitment process is complex and often inefficient. Previous studies have estimated that only 2% to 8% of adult patients with cancer are enrolled in clinical trials.1 Furthermore, 40% of cancer-related clinical trials terminate prematurely, with poor accrual rates cited as one of the main reasons for premature trial closure.2
Patient-trial matching through eligibility screening is a critical step in research recruitment which is usually performed by research staff. Nevertheless, it is a labor-, resource-, and time-intensive process that requires many hours for a single evaluation and additional time when multiple trials are available and/or when there are changes in patients’ clinical status.3 Challenges of the screening process and causes of poor accrual include limited physician awareness and knowledge of available studies, strict eligibility criteria, narrow enrollment windows, and the need for manual data extraction owing to free-text data in multiple information systems.2,4, 5, 6
Prescreening, defined as identifying potentially eligible patients using selective, limited eligibility criteria, can help to exclude ineligible patients, reduce screening burden by developing a smaller pool for manual chart review, and improve accrual rates.7, 8, 9, 10 Nevertheless, literature on prescreening interventions remain limited to single clinical trials or short-term pilots with prescreening only used for select patient groups. We describe the real-world experience of implementing a standardized, universal prescreening protocol at VA Connecticut Comprehensive Cancer Center and its impact on research recruitment to thoracic oncology studies.
Materials and Methods
VA Connecticut Comprehensive Cancer Center is a multidisciplinary cancer center serving veterans in Connecticut and Western Massachusetts and is a member of the National Cancer Institute (NCI) SWOG Cancer Research Network. We implemented a prescreening protocol at our cancer center in March 2017 (Appendix 1). All veterans undergoing a cancer workup or with a confirmed cancer diagnosis were eligible for prescreening. Research coordinators identified potential patients through our Cancer Care Tracking System (CCTS), tumor board lists, clinic and consult lists, provider referrals, and the cancer registry. CCTS has been previously described and is a home-grown application for identifying and tracking potential malignancies through radiographic and other alerts.11
The research coordinators manually entered data into Research Electronic Data Capture (REDCap), a web-based application designed for research data collection. Captured data included patient demographics, disease characteristics, previous treatment(s), patient identification source, and primary oncologist. The coordinators also entered key clinical trial eligibility criteria. REDCap then provided real-time lists of potential research studies for eligible patients on the basis of prescreening criteria and enrollment deadlines. The primary oncologist was notified of potentially eligible patients several days before their clinic appointment to allow time for more complete screening and informed consent as applicable. If patients were not currently eligible for studies, their data were updated accordingly and remained on waitlists for future consideration. Prescreening was implemented for all cancer-related studies, but we are reporting on its application in thoracic oncology, our most active clinical trials program. Lung cancer-related clinical trials open at our center postimplementation are listed in Appendix 2. Our prescreening protocol was approved by our Institutional Review Board.
Results
From March 2017 to December 2020, a total of 1518 patients with lung nodules and suspected and/or confirmed lung cancers were prescreened, with patient and disease characteristics described in Table 1. Of these patients, 54.9% were identified from CCTS, 22.3% from tumor board lists, 21.7% from clinic and consult lists, and the rest from provider referrals or the cancer registry. The median age was 72 years, 3.2% of patients were female, 85.2% of patients were white, and 11.9% were black/African American.
Table 1.
Prescreened Patient Demographics and Disease Characteristics
| Patient and Disease Characteristics | n | % | ||
|---|---|---|---|---|
| Age (mean) | 72 ± 8.3 y | |||
| Gender | Male | 1468 | 96.7 | |
| Female | 49 | 3.2 | ||
| Other | 1 | 0.1 | ||
| Ethnicity | White/Caucasian | 1293 | 85.2 | |
| Black/African American | 180 | 11.9 | ||
| Asian American or Pacific Islander | 11 | 0.8 | ||
| American Indian/Alaskan Native | 2 | 0.1 | ||
| Mixed race | 1 | 0.01 | ||
| Unknown/declined | 31 | 2.0 | ||
| Source of identification | Cancer care tracking system | 833 | 54.9 | |
| Tumor board | 338 | 22.3 | ||
| Clinic/consult | 330 | 21.7 | ||
| Provider referral | 12 | 0.8 | ||
| Cancer registry | 1 | 0.1 | ||
| Not recorded | 4 | 0.2 | ||
| Diagnosis | Confirmed lung cancer | 506 | 33.3 | |
| Suspected lung cancer (not confirmed by path) | 55 | 3.6 | ||
| Lung nodules | 957 | 63.0 | ||
| Cancer staging | NSCLC | Stage I | 181 | 41.1 |
| Stage II | 68 | 15.5 | ||
| Stage III | 92 | 20.9 | ||
| Stage IV | 79 | 17.9 | ||
| Stage unknown/not available | 20 | 4.5 | ||
| Total | 440 | |||
| SCLC | Limited | 31 | 47.0 | |
| Extensive | 33 | 50.0 | ||
| Unknown | 2 | 3.0 | ||
| Total | 66 | |||
Of the prescreened patients, 506 had a pathologically confirmed lung cancer diagnosis and 55 had a presumed early lung cancer diagnosis and were treated with radiation without pathologic confirmation. The cancer registry contained 498 patients with lung cancer diagnosed during this period, all of which were captured through prescreening. In addition, prescreening captured patients presenting with disease recurrence or progression. The remaining 957 patients in the database were being monitored for lung nodules. For patients with confirmed lung cancer, 87.0% had non-small cell lung cancer (NSCLC) and 13.0% had small cell lung cancer (SCLC).
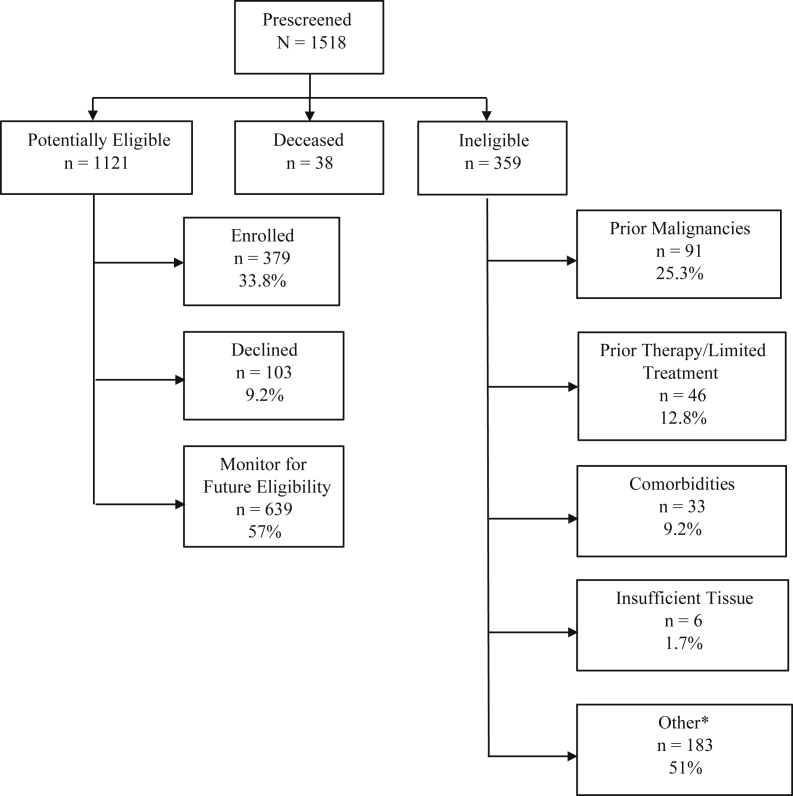
At the initial step of prescreening, 359 patients were found to be ineligible, 38 patients were deceased, and 1121 patients were identified as potential study candidates (Fig. 1). Causes for exclusion included previous malignancies (25.3%), previous treatments (12.8%), comorbidities (9.2%), insufficient tissue (1.7%), and other reasons (51%). The latter group included poor performance status, absence of targeted mutation, timing outside eligibility window, deemed poor candidate by clinician, and others. For potential candidates, 482 were found to be fully eligible for actively enrolling studies and invited to enroll. Of these, 379 patients consented and enrolled to a study and 103 patients declined. Of 38 black/African American patients who were invited to participate, 24 agreed to participation (63.2%) and 14 declined (36.8%). In comparison, 343 of 426 (80.5%) invited white/Caucasian patients agreed to participation and 83 (19.5%) declined.
Figure 1.
Disposition of prescreened patients with lung cancer and/or lung nodules enrolled in VACT prescreening protocol March 2017 to December 2020. ∗Moved out of state, study specific criteria (e.g., outside eligibility/registration window, poor performance status, absence of targeted mutation), not a good candidate per clinician, etc. VACT, Veterans Affairs Connecticut Healthcare System.
Of the patients who enrolled to studies after prescreening implementation, 86 were enrolled to therapeutic studies, 209 to diagnostic studies, and 84 to biorepository studies (Table 2). The number of patients enrolled on therapeutic studies increased from 6 in 2015 and 13 in 2016 before the prescreening protocol, to 15 in 2017 when prescreening was initiated, to 23 in 2018, 26 in 2019, and 22 in 2020 despite the coronavirus disease 2019 pandemic. In addition, 209 patients were enrolled to a lung nodule liquid biopsy study during this period.
Table 2.
Number of Patients Enrolled to Thoracic Oncology Research Studies 2015 to 2020
| Study Type | 2015 | 2016 | 2017 (Prescreening Initiation) | 2018 | 2019 | 2020 | Total Since Prescreening Initiation |
|---|---|---|---|---|---|---|---|
| Therapeutic studies | 6 | 13 | 15 | 23 | 26 | 22 | 86 |
| Diagnostic screening | 0 | 0 | 61 | 99 | 40 | 9 | 209 |
| Biorepositories | 12 | 26 | 8 | 23 | 24 | 29 | 84 |
| Total enrolled | 18 | 39 | 84 | 145 | 90 | 60 | 379 |
| New lung cancer cases in cancer registry | 128 | 135 | 156 | 122 | 124 | 96 | |
| Unique lung cancer clinic visits | 456 | 302 | 331 | 330 | 344 | 340 | |
| Total lung cancer clinic visits | 1966 | 1676 | 2005 | 2123 | 1677 | 1822 |
Discussion
We demonstrate that a universal prescreening protocol at a VA Cancer Center was associated with improved patient enrollment to thoracic oncology research studies. Our prescreening protocol captured all patients with lung cancer in the cancer registry, showing that it was a comprehensive and reliable method to identify potential research subjects. As part of our prescreening protocol, we created a centralized, customizable database in REDCap with key patient and clinical trial data, allowing us to monitor patients prospectively and continuously for clinical trial eligibility and cultivate a waitlist for future trials. For example, patients undergoing curative-intent surgery are considered for adjuvant studies, such as Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial (ALCHEMIST), and, on disease recurrence, for studies such as INSIGNA and Lung-MAP.
To the best of our knowledge, we are the only VA facility using a comprehensive prescreening protocol, and we believe that this process enabled our medium-sized cancer center to be the highest enrolling VA to NCI National Clinical Trials Network (NCTN) clinical trials since 2019. Furthermore, among all sites (VA and non-VA), we were in the top 10 enrolling sites to the Lung-MAP and ALCHEMIST CHEMO-IO NCTN studies in 2021.
Universal, standardized prescreening for clinical trials addresses several known challenges in the enrollment process. Prescreening shares the responsibility of patient-trial matching between providers and research coordinators, therefore decreasing missed opportunities from unawareness of trial availability or narrow enrolment windows, patient exclusion on the basis of provider preconceptions, and screen failures. We found that our prescreening protocol helped avoid last-minute scrambles in busy clinics during which providers review eligibility criteria and locate the correct consent form. There is also increasing recognition that a lack of diverse recruitment limits research generalizability, with recruitment of racial/ethnic minorities persistently lagging for cancer trials.12 Standardized prescreening ensures that all eligible patients are identified irrespective of their ethnicity and psychosocial status. Unfortunately, our prescreening protocol revealed that invited black/African American patients were almost twice as likely to decline participation compared with invited white/Caucasian patients. This reveals that universal prescreening is only the initial step in improving trial diversity and that additional tools, such as patient navigators and community partnerships, are needed to increase minority participation.12
The clinical trial phase is the most expensive component of drug development, with the estimated cost for the screening and enrollment process to be several hundreds to thousands of dollars per enrolled patient.3 The NCI Clinical Trial Cooperative Group Program guidelines for clinical trial systems recommends developing a robust, standardized, and accessible clinical trial infrastructure with a complete database of active and planned trials and standardized electronic data capture.13 On the basis of our real-world experience and outcomes, we believe that our prescreening protocol meets the NCI guidelines by using a centralized research recruitment system. Although many academic disease-focused clinics may already have an informal prescreening process, our formalized and Institutional Review Board–approved protocol builds a current and readily modifiable database and is also applicable to community-based clinical settings similar to our own.
We recognize that our prescreening protocol is labor intensive, and we anticipate that future artificial intelligence systems may automate many of our processes. For example, a previous retrospective study revealed that a clinical decision support system could accurately determine trial eligibility for patients with breast cancer.14 Nevertheless, many such systems are not yet mature, have high false-positive rates, and demonstrate the current necessity of manual review for a reliable process.15 Our work reveals that a mostly manual prescreening protocol increases enrollment to lung cancer clinical trials, and we believe that our methods can provide guidance, input, and validation as decision support tools mature.
CRediT Authorship Contribution Statement
Jenny Xiang: Formal analysis, Investigation, Visualization, Roles/Writing - original draft, Writing - review & editing.
Alicia Roy, Christine Summers, Monica Delvy: Data curation, Methodology, Project administration.
Jessica O’Donovan, John Christensen, Christopher Dwy, Lydia Perry, Donna Connery: Data curation.
Michal G. Rose: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing - review & editing.
Kelsey Sheehan: Formal analysis, Investigation, Methodology, Writing - review & editing.
Herta Chao: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing - review & editing, Supervision.
Footnotes
Disclosure: This work was supported by the National Institutes of Health R01 grant CA218501 to Dr. Chao; VA Merit Review Grant CX001301 to Dr. Chao; NCI and VA Interagency Group to Accelerate Trials Enrollment grant to Dr. Chao; and the Lung Precision Oncology Program to Dr. Rose. Drs. Rose, Sheehan, and Chao report receiving standard funding from Bristol Myers Squibb, Janssen to VA Connecticut Research & Education Foundation to support patients enrolled on their sponsored studies. The remaining authors declare no conflict of interest.
Cite this article as: Xiang JJ, Roy A, Summers C, et al. Brief report: implementation of a universal prescreening protocol to increase recruitment to lung cancer studies at a Veterans Affairs Cancer Center. JTO Clin Res Rep. 2022;3:100357.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100357.
Supplementary Data
References
- 1.Unger J.M., Vaidya R., Hershman D.L., Minasian L.M., Fleury M.E. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. JNCI J Natl Cancer Inst. 2019;111:245–255. doi: 10.1093/jnci/djy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain N.M., Culley A., Knoop T., Micheel C., Osterman T., Levy M. Conceptual framework to support clinical trial optimization and end-to-end enrollment workflow. JCO Clin Cancer Inform. 2019;3:1–10. doi: 10.1200/CCI.19.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penberthy L.T., Dahman B.A., Petkov V.I., DeShazo J.P. Effort required in eligibility screening for clinical trials. J Oncol Pract. 2012;8:365–370. doi: 10.1200/JOP.2012.000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lara P.N., Jr., Higdon R., Lim N., et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 5.Baggstrom M.Q., Waqar S.N., Sezhiyan A.K., et al. Barriers to enrollment in non-small cell lung cancer therapeutic clinical trials. J Thorac Oncol. 2011;6:98–102. doi: 10.1097/JTO.0b013e3181fb50d8. [DOI] [PubMed] [Google Scholar]
- 6.Garcia S., Bisen A., Yan J., et al. Thoracic oncology clinical trial eligibility criteria and requirements continue to increase in number and complexity. J Thorac Oncol. 2017;12:1489–1495. doi: 10.1016/j.jtho.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thadani S.R., Weng C., Bigger J.T., Ennever J.F., Wajngurt D. Electronic screening improves efficiency in clinical trial recruitment. J Am Med Inform Assoc. 2009;16:869–873. doi: 10.1197/jamia.M3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L., Grant J., Cheung W.Y., Kennecke H.F. Screening intervention to identify eligible patients and improve accrual to phase II-IV oncology clinical trials. J Oncol Pract. 2013;9:e174–e181. doi: 10.1200/JOP.2012.000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Embi P.J., Jain A., Clark J., Bizjack S., Hornung R., Harris C.M. Effect of a clinical trial alert system on physician participation in trial recruitment. Arch Intern Med. 2005;165:2272–2277. doi: 10.1001/archinte.165.19.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J., Yakubov A., Abdul-Hay M., et al. Prescreening to increase therapeutic oncology trial enrollment at the largest public hospital in the United States. JCO Oncol Pract. 2022;18:e620–e625. doi: 10.1200/OP.21.00629. [DOI] [PubMed] [Google Scholar]
- 11.Alsamarai S., Yao X., Cain H.C., et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer. 2013;14:527–534. doi: 10.1016/j.cllc.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Vuong I., Wright J., Nolan M.B., et al. Overcoming barriers: evidence-based strategies to increase enrollment of underrepresented populations in cancer therapeutic clinical trials—a narrative review. J Cancer Educ. 2020;35:841–849. doi: 10.1007/s13187-019-01650-y. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine (US) Committee on Cancer Clinical Trials and the NCI Cooperative Group Program . In: A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Nass S.J., Moses H.L., Mendelsohn J., editors. The National Academies Press; Washington, DC: 2010. [PubMed] [Google Scholar]
- 14.Haddad T., Helgeson J.M., Pomerleau K.E., et al. Accuracy of an artificial intelligence system for cancer clinical trial eligibility screening: retrospective pilot study. JMIR Med Inform. 2021;9 doi: 10.2196/27767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain N.M., Culley A., Micheel C.M., Osterman T.J., Levy M.A. Learnings from precision clinical trial matching for oncology patients who received NGS testing. JCO Clin Cancer Inform. 2021;5:231–238. doi: 10.1200/CCI.20.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.