Abstract
Obstructive sleep apnea (OSA) is a common sleep disorder that has been associated with cardiovascular consequences. Rapid eye movement (REM)-related obstructive sleep apnea (OSA) is a subtype of OSA which is characterized by apneas or hypopneas predominately during REM sleep. The factors associated with REM-related OSA are still unclear. We aimed to determine the prevalence and associated characteristics of REM-related OSA in Thai patients. A total of 408 patients’ charts were retrospectively reviewed. Demographic and anthropometric characteristics, comorbidities and polysomnographic data were obtained. The patients were divided into two groups: REM-related OSA and non-stage specific OSA. REM-related OSA was defined as an apnea–hypopnea index (AHI) ≥ 5 per hour, with a ratio of REM-AHI to NREM-AHI > 2, and NREM-AHI < 15 per hour. The prevalence of REM-related OSA was 21.6%. AHI and arousal index were both lower in REM-related OSA than in non-stage specific OSA. REM-related OSA was significantly associated with females (OR 2.35, 95% CI 1.25–4.42, p = 0.008), age < 60 years (OR 2.52, 95% CI 1.15–5.55, p = 0.021), and mild OSA (OR 17.46, 95% CI 9.28–32.84, p < 0.001). In conclusion, age < 60 years, female gender, and mild severity of OSA were associated with REM-related OSA.
Subject terms: Health care, Medical research, Risk factors
Introduction
Obstructive sleep disorder (OSA) is characterized by repetitive narrowing and collapse of the upper airway, resulting in decreased or complete cessation of airflow during sleep1, which leads to recurring episodes of hypoxemia, hypercapnia, sleep fragmentation, and increased sympathetic activity2. It is a common sleep disorder, affecting between 9 and 38% of adults in the general population3.
OSA is a complex heterogeneous disorder so apnea–hypopnea index (AHI) alone cannot discriminate the severity or variety of these conditions4. Many categories or phenotypes of patients with OSA have been proposed to classify patients into more homogenous categories. In particular, the distribution of respiratory events between sleep stages of rapid eye movement (REM) sleep and non-rapid eye movement (NREM) sleep are used to categorize OSA patients.
REM-related OSA is characterized by respiratory events such as apnea and hypopnea predominately during REM sleep5. The estimated prevalence of REM-related OSA has been reported to be between 10 and 36%6,7 of patients with obstructive sleep apnea and it varies according to subjects’ characteristics, and definitions used. In general, the common criteria that is used to identify REM-related OSA is an overall AHI of ≥ 5 and a ratio of the AHI during REM sleep to the AHI during NREM sleep of ≥ 25. Patients with REM-related OSA tend to be younger individuals, women7,8 and patients with mild or moderate OSA6,9.
REM-related OSA is independently associated with cardiovascular and metabolic complications such as hypertension10, metabolic syndrome and diabetes11, but the treatment of REM-related OSA is not standardized and patients with this condition may be untreated or undertreated.
Many studies have been published concerning this subtype of OSA, however the factors associated with REM-related OSA remain unclear and no study has reported the prevalence of REM-related OSA in Thai populations. Therefore, the aim of this study was to evaluate the prevalence and to explore characteristics of REM-related OSA in Thailand.
Material and methods
Ethics statement
This study was approved by The Human Research Ethics Committee of Thammasat University (Medicine) (IRB project No. 008/2564), in compliance with Declaration of Helsinki, The Belmont Report, CIOMS guidelines and Good Clinical Practice (ICH-GCP) guidelines. All methods were performed in accordance with these guidelines and regulations. All participants provided written informed consent.
Study design and subjects
This was retrospective chart review study. We included all patients who were over 18 years old with suspected OSA and underwent standard diagnostic polysomnography (either split-night or full-night) between November 2019 and October 2020. The study was conducted at sleep lab, Thammasat University Hospital, Thammasat University, Pathum Thani, Thailand. The suspected OSA patients were assessed by symptoms suggesting OSA, such as snoring, observed apnea, nocturnal choking, nocturia, and daytime sleepiness from the request document for PSG. Patients were excluded from the study if AHI < 5 per hour, total sleep time < 100 min, REM sleep duration < 10.5 min, or polysomnographic data was incomplete. Figure 1 shows the study population flow chart.
Figure 1.
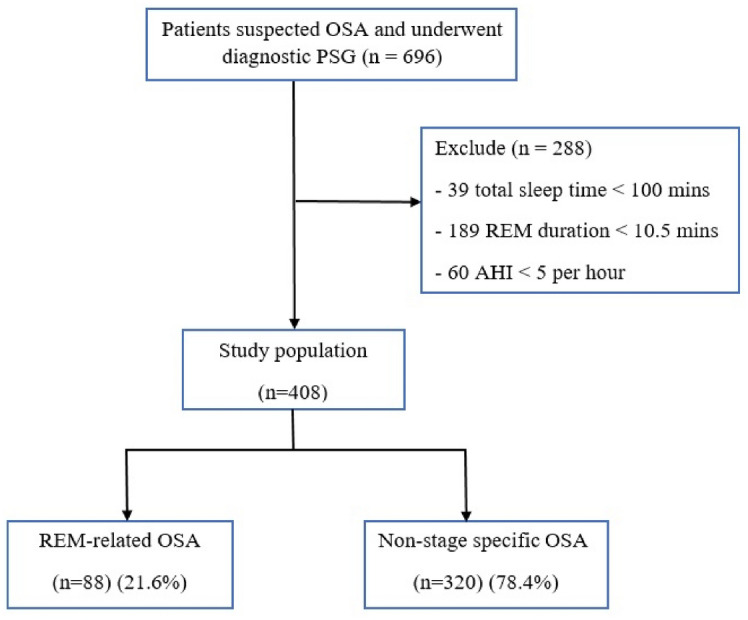
Study population flow chart. OSA obstructive sleep apnea, PSG polysomnography, AHI apnea–hypopnea index, REM rapid eye movement.
Demographic data (age, sex), self-reported questionnaire, Thai Epworth sleepiness scale and anthropometric measurement were routinely recorded prior to PSG.
The comorbidities were obtained from medical records or current drug treatment.
Anthropometric measurements were taken by trained sleep technicians using standard techniques12. Weight in kilograms (kg) was measured while wearing light clothing and without shoes, using a digital scale to the nearest 100 g. Height in meters (m) was measured without shoes, with stadiometer to the nearest 0.5 cm (cm). Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). The patients were categorized into 2 groups according to the Asian-Pacific cutoff value13 for obesity, which was ≥ 25 kg/m2. Neck circumference (NC) was measured (cm) using an inelastic tape with 1 mm precision at the level of the cricothyroid membrane in upright position14. The cutoff point 40 cm was used for large NC.
Daytime sleepiness was evaluated using the Thai version of the Epworth Sleepiness Scale (ESS) questionnaires15. A score > 10 was considered to indicate excessive daytime sleepiness.
Polysomnography and scoring
A standard polysomnography (PSG) was performed according to American Academy of Sleep Medicine (AASM) recommendations16 by using a digital polysomnographic monitor (Greal Series, Compumedics, Victory, Australia). The following biological variables were monitored continuously: electroencephalography (F4-M1, C4-M1, O2-M1, F3-M2, C3-M2 and O1-M2), electrooculography, submental and anterior tibial electromyography, and electrocardiography. Inductance plethysmography belts were used to monitor chest and abdominal movements. Thermistor and nasal pressure transducers were used to monitor airflow. Snoring was recorded using a microphone. Pulse oximetry was used to measure arterial oxygen saturation (SpO2). The cumulative percentage of time spent at saturation below 90% was calculated from pulse oximetry. Data were scored manually by a sleep specialist according to the AASM scoring system16.
Apnea was defined as a ≥ 90% reduction of airflow with a duration of at least 10 s, while hypopnea was defined as a ≥ 30% reduction of airflow that was associated with either arousal or with a ≥ 3% arterial oxygen desaturation, for a duration of at least 10 s.
AHI was calculated as the mean number of events of either apnea or hypopnea per hour of total sleep time. REM-AHI was the mean number of events, either apnea or hypopnea, per hour of REM sleep. NREM-AHI was the mean number of events, either apnea or hypopnea, per hour of NREM sleep.
Definitions
The diagnosis of OSA was made by evidence of AHI ≥ 5. The OSA severity was classified as: mild for 5 ≤ AHI < 15, moderate for 15 ≤ AHI < 30, and severe for AHI ≥ 3017.
Patients were considered to have REM-related OSA if the ratio of REM-AHI to NREM-AHI was > 2 and NREM-AHI < 158. Patients who did not fulfill the definition of REM-related OSA were considered to have non-stage specific OSA. After sorting the patients into these two groups, the demographic and anthropometric characteristics, comorbidities, and polysomnographic characteristics of the REM-related OSA group and the non-stage specific OSA group were compared.
Statistical analysis
The data was analyzed using Stata version 14 (STATA Corp., Texas, USA). The prevalence is reported as percentage of population. Continuous variables are presented as mean ± standard deviation for normally distributed variables or median and interquartile range (IQR) for non-normally distributed variables. Categorical variables are presented as number and percentage. Comparisons between groups were analyzed using the unpaired t test for continuous variables with normal distribution, the Mann–Whitney U test for continuous variables with non-normal distribution, and the Chi-square test for categorical variables.
Potential associated factors of REM-related OSA were analyzed using multivariate logistic regression analysis to calculate the odds ratios (ORs) and 95% confidence intervals (CIs). Demographic and clinical variables including age, gender, NC, BMI, excessive daytime sleepiness, hypertension, asthma, diabetes and OSA severity were examined in the univariate analysis. All variables on univariate analysis were also used for analysis in the multivariate logistic regression model. All statistical tests were two-sided, and p-value < 0.05 was considered to be statistically significant.
Results
Prevalence of REM-related OSA
Of the 696 patients suspected of OSA who underwent diagnostic PSG, 288 patients were excluded from study and 408 patients were enrolled and diagnosed as OSA (Fig. 1). The participants consisted of 245 (60.1%) men and 163 (39.9%) women; their median age was 49.7 ± 15.8 years.
The prevalence of REM-related OSA was 21.6%. REM-related OSA was more prevalent in younger patients (45.2 ± 14.9 vs. 50.9 ± 15.8, p = 0.002) and in women (54.5% vs. 35.9%, p = 0.002).
Comparison of demographic, anthropometric, ESS, comorbidities characteristics between patients with REM-related OSA and patients with non-stage specific OSA
The REM-related OSA group of patients tended to have thinner necks than the non-stage specific OSA group; (75% vs. 56.3%, p = 0.001) had neck circumferences ≤ 40 cm. The non-stage specific OSA group had a higher proportion of comorbid diabetes (25.9% vs. 13.6%, p = 0.016). There were no significant differences in BMI, Epworth sleepiness scale, or excessive daytime sleepiness between the two groups, as shown in Table 1.
Table 1.
Demographic, anthropometric, ESS, comorbidities characteristics of patients with REM-related OSA and non-stage specific OSA.
| Characteristics | Total (n = 408) |
REM-related OSA (n = 88) |
Non-stage specific OSA (n = 320) |
p-value |
|---|---|---|---|---|
| Age, years | 49.7 ± 15.8 | 45.2 ± 14.9 | 50.9 ± 15.8 | 0.002 |
| Age | 0.004 | |||
| < 60 years | 288 (70.6) | 73 (82.9) | 215 (67.2) | |
| ≥ 60 years | 120 (29.4) | 15 (17.1) | 105 (32.8) | |
| Gender | 0.002 | |||
| Male | 245 (60.1) | 40 (45.5) | 205 (64.1) | |
| Female | 163 (39.9) | 48 (54.5) | 115 (35.9) | |
| Neck circumferences | 0.001 | |||
| ≤ 40 cm | 246 (60.3) | 66 (75.0) | 180 (56.3) | |
| > 40 cm | 162 (39.7) | 22 (25.0) | 140 (43.7) | |
| BMI classification | 0.239 | |||
| < 25 kg/m2 | 88 (21.6) | 23 (26.1) | 65 (20.3) | |
| ≥ 25 kg/m2 | 320 (78.4) | 65 (73.9) | 255 (79.7) | |
| ESS | 8.7 ± 4.9 | 8.4 ± 5.3 | 8.8 ± 4.8 | 0.462 |
| Excessive daytime sleepiness | 0.338 | |||
| No | 138 (33.8) | 26 (29.5) | 112 (35.0) | |
| Yes | 270 (66.2) | 62 (70.5) | 208 (65.0) | |
| Comorbidities | ||||
| Hypertension | 208 (51.0) | 44 (50.0) | 164 (51.2) | 0.835 |
| Diabetes mellitus | 95 (23.3) | 12 (13.6) | 83 (25.9) | 0.016 |
| Asthma | 12 (2.9) | 1 (1.1) | 11 (3.4) | 0.475 |
| COPD | 2 (0.5) | 0 (0.0) | 2 (0.6) | > 0.999 |
| Cardiovascular disease | 64 (15.7) | 9 (10.2) | 55 (17.2) | 0.112 |
| Pulmonary hypertension | 15 (3.7) | 1 (1.1) | 14 (4.4) | 0.209 |
Data are expressed as mean ± SD, or n (%).
REM rapid eye movement, OSA obstructive sleep apnea, BMI body mass index, ESS Epworth Sleepiness Scale.
Comparison of polysomnographic characteristics between patients with REM-related OSA and patients with non-stage specific OSA
Patients with REM-related OSA showed higher sleep efficiency, percentage of stage N3, mean SpO2 and minimum SpO2 than patients with non-stage specific OSA. They also showed lower percentage of stage N1, arousal index, and duration of SpO2 < 90% (Table 2).
Table 2.
Polysomnographic features of patients with REM-related OSA and non-stage specific OSA.
| Variables | Total (n = 408) |
REM-related OSA (n = 88) |
Non-stage specific OSA (n = 320) |
p-value |
|---|---|---|---|---|
| Sleep efficiency (%) | 78.9 (66.2, 87.0) | 83.7 (73.1, 89.9) | 77.9 (63.9, 86.2) | < 0.001 |
| Stage N1 (%) | 16.0 (11.0, 25.0) | 11.8 (6.7, 14.0) | 18.0 (12.0, 27.0) | < 0.001 |
| Stage N2 (%) | 49.6 ± 11.7 | 51.4 ± 11.8 | 49.1 ± 11.2 | 0.110 |
| Stage N3 (%) | 16.0 (6.7, 25.0) | 20.3 (11.5, 29.7) | 15.3 (5.8, 23.9) | < 0.001 |
| Stage REM duration (min) | 27.5 (17.0, 48.5) | 43 (22.0, 70.5) | 26 (17.0, 41.5) | < 0.001 |
| Stage REM (%) | 14.3 ± 5.6 | 15.4 ± 5.8 | 14.0 ± 5.5 | 0.047 |
| AHI (events/h) | 29.2 (14.7, 54.0) | 11.3 (7.9, 15.7) | 39.9 (22.7, 63.5) | < 0.001 |
| PSG severity | < 0.001 | |||
| Mild | 103 (25.3) | 62 (70.4) | 41 (12.8) | |
| Moderate | 107 (26.2) | 26 (29.6) | 81 (25.3) | |
| Severe | 198 (48.5) | 0 (0.0) | 198 (61.9) | |
| REM-AHI (events/h) | 45.4 (22.6, 65.1) | 37.6 (24.7, 54.6) | 49.1 (19.9, 67.9) | 0.029 |
| NREM-AHI (events/h) | 27.3 (12.0, 53.4) | 7.1 (4.4, 10.8) | 36.7 (20.7, 61.9) | < 0.001 |
| Apnea index (events/h) | 2.1 (0.2, 9.9) | 0.3 (0, 1.6) | 3.5 (0.4, 16.7) | < 0.001 |
| Hypopnea index (events/h) | 20.3 (10.5, 35.6) | 9.9 (6.8, 13.7) | 26.1 (16.1, 39.7) | < 0.001 |
| Arousal index (events/h) | 24.6 (13.9, 40.5) | 10.5 (5.8, 14.9) | 29.5 (18.1, 47.0) | < 0.001 |
| Mean SpO2 (%) | 95.0 (92.0, 96.0) | 96.0 (94.0, 97.0) | 94.0 (91.0, 96.0) | < 0.001 |
| Lowest SpO2 (%) | 83.0 (72.5, 89.0) | 86.0 (79.0, 89.0) | 82.5 (69.0, 89.0) | 0.002 |
| SpO2 time < 90% (min) | 3.0 (0.3, 12.6) | 0.9 (0.2, 3.8) | 4.3 (0.4, 18.0) | < 0.001 |
Data are expressed as mean ± SD, median (IQR), or n (%).
REM rapid eye movement, NREM non-rapid eye movement, OSA obstructive sleep apnea, AHI apnea–hypopnea index, SpO2 arterial oxygen saturation.
AHI was significantly lower in patients with REM-related OSA compared with non-stage specific OSA patients (11.3 (7.9, 15.7) vs. 39.9 (22.7, 63.5), p < 0.001). In REM-related OSA patients, mild OSA and moderate OSA were reported in 70.4% and 29.6% of patients, while no severe OSA events were reported.
Regression analysis of factors associated with REM-related OSA
The results of logistic regression analysis are shown as Table 3. According to the univariate analysis, the factors associated with REM-related OSA were female gender, age ≤ 60 years, neck circumference ≤ 40 cm, mild OSA, and diabetes. After multivariate analysis, REM-related OSA was significantly statistically associated with female gender (aOR 2.35, 95% CI 1.25–4.42, p = 0.008), age < 60 years (aOR 2.52, 95% 1.15–5.55, p = 0.021), and mild OSA (aOR 17.46, 95% CI 9.28–32.84, p < 0.001). Therefore, asthma and diabetes had negative association with REM-related OSA.
Table 3.
Regression analysis of factors associated with REM-related OSA.
| Variables | Univariate | p-value | Multivariate | p-value |
|---|---|---|---|---|
| OR (95% CI) | aOR* (95% CI) | |||
| Age < 60 years | 2.38 (1.30–4.34) | 0.005 | 2.52 (1.15–5.55) | 0.021 |
| Female | 2.14 (1.33–3.45) | 0.002 | 2.35 (1.25–4.42) | 0.008 |
| NC ≤ 40 cm | 2.33 (1.37–3.97) | 0.002 | 1.32 (0.65–2.71) | 0.443 |
| BMI ≥ 25 kg/m2 | 0.72 (0.42–1.25) | 0.241 | 1.33 (0.62–2.85) | 0.457 |
| No excessive daytime sleepiness (ESS ≤ 10) | 1.28 (0.77–2.14) | 0.339 | 1.14 (0.60–2.17) | 0.680 |
| Hypertension | 0.95 (0.59–1.52) | 0.835 | 1.59 (0.82–3.09) | 0.169 |
| Asthma | 0.32 (0.04–2.54) | 0.282 | 0.10 (0.01–0.95) | 0.045 |
| Diabetes | 0.45 (0.23–0.87) | 0.018 | 0.39 (0.16–0.95) | 0.039 |
| OSA severity | ||||
| Mild | 16.23 (9.24–23.50) | < 0.001 | 17.46 (9.28–32.84) | < 0.001 |
| Moderate to severe | Reference | Reference | ||
NC neck circumferences, BMI body mass index, ESS Epworth Sleepiness scale.
*Adjusted odds ratio obtained from multivariate logistic regression adjusting for all variables presented in this table.
Discussion
This present study was designed to determine the prevalence and associated factors including demographic and polysomnographic features of REM-related OSA in Thai patients. The results of this study showed that the prevalence of REM-related OSA was 21.6%. REM-related OSA was more prevalent in younger patients and in women.
Previous studies have considered the prevalence of REM-related OSA, but estimates vary from between 10 and 36%6,7 of OSA patients, partly because definitions of REM-related OSA differ. The most common criterion for REM-related OSA is a ratio of AHI during REM sleep to AHI during NREM sleep of ≥ 25. However, this definition may misdiagnose patients who are significantly affected by OSA during NREM if the ratio of REM-AHI to NREM-AHI is greater than 2. Therefore, we added the condition that NREM-AHI < 15. This approach has also proven clinically useful in some previous studies8,18. The prevalence of REM-related OSA in this study was equivalent to that in previous studies5,18 which used the same definition.
In this study, comparing REM-related OSA with non-stage specific OSA showed that the patients with REM-related OSA were younger individuals and more often female. Likewise, the logistic regression confirmed that age < 60 years and female gender were independent correlates of REM-related OSA. These results are consistent with other studies5,7,19.
However, the pathophysiology has not been well clarified. A possible explanation may relate to gender differences in obstructive sleep apnea such that the obstructive events are predominant in men in NREM and in women in REM. Compared to men, women have different upper airway anatomy, non-anatomical factors and sex hormones. Impaired upper airway anatomy is the key cause of OSA. The upper airway is stiffer in women than in men, whereas the pharyngeal airway is longer and there is more soft tissue thickening on the lateral pharyngeal wall in men than women, so women are less susceptible to airway collapse20.
The results of a large cohort study suggest that gender differences in OSA are influenced by state-specific mechanisms. Women demonstrated lower loop gain and lower arousal threshold in NREM21. In addition, female hormones (progesterone) may increase the upper airway dilator muscle tone and stimulate ventilation by increasing the chemoreceptor response to hypoxia and hypertonia22. But protective mechanisms in NREM do not substantively protect women from airway collapse during REM sleep which is characterized by muscle atonia and decreased chemosensitivity23.
REM-related OSA patients tend to have lower overall AHI than non-stage specific OSA patients. In the REM-related group, 70.4% of patients had mild OSA, 29.6% had moderate OSA, and none had severe OSA. In the non-stage specific group, 61.9% had severe OSA. REM sleep accounts for approximately a quarter of total sleep duration2, so the overall AHI predominantly reflects NREM-AHI. Additionally, the polysomnographic findings showed that sleep quality was better and arousal index was lower in REM-related OSA than in non-stage specific OSA, which is more severe. Increased BMI has been reported to increase severity of OSA24, but our study found no significance differences related to BMI.
Excessive daytime sleepiness is considered an important symptom of OSA. In previous studies, the association of excessive daytime sleepiness and REM-related OSA was equivocal. The current study showed no significant differences of excessive daytime sleepiness between patients with REM-related OSA and those with non-stage specific OSA, based on Epworth Sleepiness Scale, despite lower overall AHI in REM-related OSA. Interruption of REM sleep may have more impact on daytime sleepiness than interruption of NREM sleep25. Therefore, although REM-AHI in non-stage specific OSA was significantly higher, both groups showed severe REM-AHI (REM-AHI ≥ 30 per hour) which could reflect similar symptoms. Oxygen desaturation during REM sleep might cause excessive daytime sleepiness19, but our data did not show any difference.
Two population-based studies demonstrated significant and clinically relevant associations between REM-AHI and prevalent hypertension. NREM-AHI was not associated with hypertension after adjusting for REM-AHI10,26. The HypnoLaus Sleep Cohort study verified that REM-AHI ≥ 20 per hour was associated with increased risk of hypertension27. The strong association of hypertension with adverse cardiovascular outcomes link REM-related OSA to cardiovascular outcomes28. Interestingly, our data showed REM-AHI ≥ 20 per hour in both groups, so the potential of hypertension and cardiovascular disease was similar in both REM-related OSA and non-stage specific OSA.
In contrast, diabetes and asthma showed negative association with REM-related OSA. However, the results must be interpreted with caution because our study reported that diabetes and asthma were more common in non-stage specific OSA.
Plausible pathophysiological and causal links between OSA and glucose metabolism dysregulation are sleep fragmentation and intermittent hypoxemia29. In our study, non-stage specific OSA showed higher overall AHI, REM-AHI, arousal index (which represented sleep fragmentation) and oxygen desaturation.
The Sleep Heart Heath Study demonstrated that increased REM-AHI was independently associated with insulin resistance after adjustment for multiple potential confounders11 and suggested that REM-related OSA is likely to have an impact on glucose metabolism and diabetes, even absence of NREM-AHI. In addition, the relation between REM-related OSA and diabetes relied on the severity of REM-AHI. The HypnoLaus Sleep Cohort study showed REM-AHI ≥ 20 per hour or increasing REM-AHI severity were significantly associated with diabetes in both subgroups with NREM-AHI < 10 per hour and AHI < 10 per hour27.
The association between REM-related OSA and asthma was interesting because a prospective observational study showed that asthma was independently associated with REM-related OSA19, which was consistent with a previous study in children with OSA31, while our results showed a negative relationship. However, the published pathophysiologic mechanisms that explain the association between asthma and OSA, especially REM-related OSA, are not well understood and are limited. Asthma and OSA are heterogeneous conditions which exhibit complex bidirectional interactions, shared risk factors and co-morbidities between each other32.
There may be some possible limitations in this study. Firstly, in our routine practice, split-night PSG was applied more frequently than full-night PSG because it costs less.
Therefore, the duration of the REM sleep, which is mostly concentrated in the second half of the sleep period, was lower in split-night PSG than in full-night PSG33. In fact, the standard diagnostic criteria did not clarify minimum duration for REM sleep. To reduce this bias, we selected 10.5 min as the minimum REM sleep duration, but a longer REM duration may diagnose REM-related OSA more precisely. Secondly, we used moderate and severe OSA as reference in regression analysis because there was no severe OSA in the REM-related group. Our result showed mild severity of OSA was the independent associated factor. However, moderate severity was also found in REM-related OSA.
The contribution of this study is to confirm that REM-related OSA is highly prevalent in Thai OSA patients and has clinical implications related to sleepiness and comorbidities. Further studies are required to better understand the roles of asthma and diabetes in REM-related OSA. Moreover, impacts and options of therapy should be investigated.
In summary, this study has determined that age < 60 years, female gender, and mild severity of OSA were associated with REM-related OSA.
Acknowledgements
This study was supported by the research group in Clinical Epidemiology, Faculty of Medicine, Thammasat University. The authors would like to thank Michael Jan Everts for proofreading this manuscript and the sleep lab at Thammasat University Hospital for polysomnographic data.
Author contributions
N.S. and W.W. drafted the study protocol and collected data, W.W. and P.K. interpreted and analyzed data, N.S. wrote the first draft of this manuscript. W.W. and P.K. reviewed the final manuscript. All authors read, approved and agreed on the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eckert DJ. Phenotypic approaches to obstructive sleep apnoea - New pathways for targeted therapy. Sleep Med. Rev. 2018;37:45–59. doi: 10.1016/j.smrv.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Alzoubaidi M, Mokhlesi B. Obstructive sleep apnea during rapid eye movement sleep: clinical relevance and therapeutic implications. Curr. Opin. Pulm. Med. 2016;22:545–554. doi: 10.1097/MCP.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senaratna CV, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med. Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Zinchuk AV, Gentry MJ, Concato J, Yaggi HK. Phenotypes in obstructive sleep apnea: a definition, examples and evolution of approaches. Sleep Med. Rev. 2017;35:113–123. doi: 10.1016/j.smrv.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conwell W, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16:519–526. doi: 10.1007/s11325-011-0537-6. [DOI] [PubMed] [Google Scholar]
- 6.Haba-Rubio J, Janssens JP, Rochat T, Sforza E. Rapid eye movement-related disordered breathing: clinical and polysomnographic features. Chest. 2005;128:3350–3357. doi: 10.1378/chest.128.5.3350. [DOI] [PubMed] [Google Scholar]
- 7.Koo BB, Dostal J, Ioachimescu O, Budur K. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath. 2008;12:259–264. doi: 10.1007/s11325-007-0161-7. [DOI] [PubMed] [Google Scholar]
- 8.Sakao S, et al. Features of REM-related sleep disordered breathing in the Japanese population. Intern. Med. 2015;54:1481–1487. doi: 10.2169/internalmedicine.54.4248. [DOI] [PubMed] [Google Scholar]
- 9.Sunnetcioglu A, Sertogullarından B, Ozbay B, Gunbatar H, Ekin S. Obstructive sleep apnea related to rapid-eye-movement or non-rapid-eye-movement sleep: comparison of demographic, anthropometric, and polysomnographic features. J. Bras. Pneumol. 2016;42:48–54. doi: 10.1590/S1806-37562016000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appleton SL, et al. Hypertension is associated with undiagnosed OSA during rapid eye movement sleep. Chest. 2016;150:495–505. doi: 10.1016/j.chest.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Chami HA, Gottlieb DJ, Redline S, Punjabi NM. Association between glucose metabolism and sleep-disordered breathing during REM sleep. Am. J. Respir. Crit. Care Med. 2015;192:1118–1126. doi: 10.1164/rccm.201501-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fryar CD, Gu Q, Ogden CL, Flegal KM. Anthropometric reference data for children and adults: United States, 2011–2014. Vital Health Stat. 2016;3:1–46. [PubMed] [Google Scholar]
- 13.World Health Organization. Regional Office for the Western, P. The Asia-Pacific Perspective: Redefining Obesity and its Treatment (Health Communications Australia, 2000).
- 14.Banhiran W, Junlapan A, Assanasen P, Chongkolwatana C. Physical predictors for moderate to severe obstructive sleep apnea in snoring patients. Sleep Breath. 2014;18:151–158. doi: 10.1007/s11325-013-0863-y. [DOI] [PubMed] [Google Scholar]
- 15.Banhiran W, Assanasen P, Nopmaneejumruslers C, Metheetrairut C. Epworth sleepiness scale in obstructive sleep disordered breathing: the reliability and validity of the Thai version. Sleep Breath. 2011;15:571–577. doi: 10.1007/s11325-010-0405-9. [DOI] [PubMed] [Google Scholar]
- 16.Berry RB, et al. AASM scoring manual updates for 2017 (Version 2.4) J. Clin. Sleep Med. 2017;13:665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapur VK, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasai T, Kayaba M, Inoue Y, Nakayama H. Prevalence, clinical symptoms and polysomnographic findings of REM-related sleep disordered breathing in Japanese population. Sleep Med. 2021;80:52–56. doi: 10.1016/j.sleep.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Bahammam RA, et al. The associations of gender, menopause, age, and asthma with REM-predominant obstructive sleep apnea: a prospective observational study. Nat. Sci. Sleep. 2020;12:721–735. doi: 10.2147/NSS.S275051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayub S, Won CHJ. Obstructive sleep apnea in women. J. Sleep Med. 2019;16:75–80. doi: 10.13078/jsm.190047. [DOI] [Google Scholar]
- 21.Won, C. H. J., et al. Sex differences in obstructive sleep apnea phenotypes, the multi-ethnic study of atherosclerosis. in Sleep, Vol. 43 zsz274 (Oxford University Press, 2020). [DOI] [PMC free article] [PubMed]
- 22.LoMauro, A. & Aliverti, A. Sex differences in respiratory function. in Breathe, Vol. 14 131 (2018). [DOI] [PMC free article] [PubMed]
- 23.Koo BB, Patel SR, Strohl K, Hoffstein V. Rapid eye movement-related sleep-disordered breathing: influence of age and gender. Chest. 2008;134:1156–1161. doi: 10.1378/chest.08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leong WB, et al. The prevalence and severity of obstructive sleep apnea in severe obesity: the impact of ethnicity. J. Clin. Sleep Med. 2013;09:853–858. doi: 10.5664/jcsm.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabryelska A, Białasiewicz P. Association between excessive daytime sleepiness, REM phenotype and severity of obstructive sleep apnea. Sci. Rep. 2020;10:34. doi: 10.1038/s41598-019-56478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokhlesi B, et al. Obstructive sleep apnea during REM sleep and hypertension. Results of the Wisconsin Sleep Cohort. Am. J. Respir. Crit Care Med. 2014;190:1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acosta-Castro P, et al. REM-associated sleep apnoea: prevalence and clinical significance in the HypnoLaus cohort. Eur. Respir. J. 2018;52:1702484. doi: 10.1183/13993003.02484-2017. [DOI] [PubMed] [Google Scholar]
- 28.Varga AW, Mokhlesi B. REM obstructive sleep apnea: risk for adverse health outcomes and novel treatments. Sleep Breath. 2019;23:413–423. doi: 10.1007/s11325-018-1727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152:1070–1086. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khatri SB, Ioachimescu OC. The intersection of obstructive lung disease and sleep apnea. Clevel. Clin. J. Med. 2016;83:127. doi: 10.3949/ccjm.83a.14104. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez MJ, Zhu J, Rodriguez-Martinez CE, Nino CL, Nino G. Nocturnal phenotypical features of obstructive sleep apnea (OSA) in asthmatic children. Pediatr. Pulmonol. 2013;48:592–600. doi: 10.1002/ppul.22713. [DOI] [PubMed] [Google Scholar]
- 32.Pepito DL, Mohammed JM, Hardin KA. Obstructive sleep apnea and asthma: more than chance? Curr. Pulmonol. Rep. 2021;10:84–91. doi: 10.1007/s13665-021-00271-5. [DOI] [Google Scholar]
- 33.Ciftci B, Ciftci TU, Guven SF. Split-night versus full-night polysomnography: comparison of the first and second parts of the night. Arch. Bronconeumol. 2008;44:3–7. doi: 10.1157/13114650. [DOI] [PubMed] [Google Scholar]


