Abstract
Background:
Dramatic improvements in visualization of cortical (especially subpial) MS lesions allow assessment of impact on clinical course.
Objective:
Characterize cortical lesions by 7-tesla T2*-/T1-weighted MRI; determine relationship with other MS pathology and contribution to disability.
Methods:
64 adults with MS (45 relapsing-remitting/19 progressive) underwent 3T brain/spine MRI, 7T brain MRI, and clinical testing.
Results:
Cortical lesions were found in 94% (progressive: median 56/range 2–203; relapsing-remitting: 15/0–168; p=0.004). Lesion distribution across 50 cortical regions was nonuniform (p=0.006), with highest lesion burden in supplementary motor cortex and highest prevalence in superior frontal gyrus. Leukocortical and white matter lesion volumes were strongly correlated (r=0.58, p<0.0001), while subpial and white matter lesion volumes were moderately correlated (r=0.30, p=0.002). Leukocortical (p=0.02) but not subpial lesions (p=0.40) correlated with paramagnetic-rim lesions; both were correlated with spinal cord lesions (p=0.01). Cortical lesion volume (total and subtypes) were correlated with expanded disability status scale, 25-foot timed walk, 9-hole peg test, and symbol digit modality test scores.
Conclusion:
Cortical lesions are highly prevalent and are associated with disability and progressive disease. Subpial lesion burden is not strongly correlated with white matter lesions, suggesting differences in inflammation and repair mechanisms.
Keywords: Multiple sclerosis, cortical lesions, 7-tesla MRI
Introduction
In MS, cortical lesions are common and often extensive on postmortem pathology,1–3 but until recently have been difficult to visualize by MRI. Understanding their impact on disease course and relationship with other aspects of MS is therefore limited. Of particular interest are subpial lesions, which touch the pial surface of the brain and may form via a distinct mechanism, related to meningeal inflammation,3–6 and thus may contribute independently to disability and respond differently to disease-modifying therapy. However, even advanced MRI methods at 1.5 or 3 tesla (T) are insensitive to subpial lesions.7–9
Recently, 7T T1- or T2*-weighted (w) methods have been used to study cortical lesions in small cohorts.10–15 We have demonstrated that combined use of ultra-high-resolution, optimized T1w and T2*w 7T images greatly improves visualization of cortical, including subpial, lesions.16 These methods also allow for better distinction between leukocortical and subpial lesions.
Here we apply this methodology to determine the extent and location of cortical demyelination in a cohort of 64 adults with MS. Given the potential differences in myelination, inflammation, and repair mechanisms in different CNS tissue compartments, we explore whether subpial lesions are associated with pathology in these compartments, including white matter lesions, chronic active lesions, spinal cord lesions, and leptomeningeal enhancement (LME). We also address whether and how different lesion types contribute to disability.
Materials and methods
Clinical cohort
Participants enrolled in our institutional review board-approved MS natural history study provided written, informed consent. We built a prospective subcohort of MS patients17 who were ≥18 years old and without 7T MRI contraindication. For the purpose of future longitudinal studies examining the relationship between cortical lesion formation and disability accumulation, we prospectively enrolled participants with stable white matter and spinal cord lesions by 3T MRI in the year prior to enrollment (“stable” MS), including 36 with relapsing remitting MS (RRMS) and 19 with progressive MS (PMS; 15 secondary progressive (SPMS) and 4 primary progressive (PPMS)). We also enrolled 9 participants with RRMS who had at least one new or contrast-enhancing white matter lesion in the year prior to enrollment (“active” MS). Clinical history and physical exam were obtained by MS clinicians and included expanded disability status scale (EDSS), paced auditory symbol addition test (PASAT), symbol digit modality test (SDMT, paper based), 25-foot timed walk (25TW), and 9-hole peg test (9HPT). The 25TW and 9HPT were performed at both the 3T and 7T MRI visits, and results were averaged. Multiple sclerosis severity score (MSSS) was calculated based on EDSS and disease duration.18
MRI acquisition
Each participant underwent 3T brain and cervicothoracic spine and 7T brain MRI. Median interval between 3T and 7T MRI was 9 weeks (range 1–34). 3T brain scans included axial 2D proton density (PD)/T2w, sagittal 3D magnetization prepared 2 rapid gradient echo (MP2RAGE), and sagittal 3D T2w fluid attenuated inversion recovery (FLAIR) before and after administration of gadobutrol. Spine scans included sagittal 2D short-T1 inversion recovery (STIR) and sagittal 3D T1w gradient-recalled echo (GRE), sagittal 3D MP2RAGE of the cervical spine, and axial 3D MP2RAGE of the thoracic spine. 7T brain scans included axial 3D MP2RAGE (0.5mm isometric, acquired four times per scan session), sagittal 3D segmented T2*w echo-planar imaging (EPI) (0.5mm isometric, acquired in two partially overlapping volumes for full brain coverage), and axial 2D T2*w multi-echo GRE (0.5mm isometric, acquired in three partially overlapping volumes for near full supratentorial brain coverage) (Supplementary Table 1). MP2RAGE data were processed into uniform denoised images (hereafter T1w MP2RAGE) and T1 maps using manufacturer-provided software.
Image processing and analysis
The 7T T1w and T1 map MP2RAGE repetitions were coregistered and median T1w and T1 map images were generated, as described previously.16 The 7T T2*w GRE magnitude images were averaged across echo times, and the averaged GRE images and the T2*w EPI images were linearly registered (FMRIB’s Linear Image Registration Tool, FLIRT19, 20) to the 7T T1w MP2RAGE median images. 3T images were processed and registered as described previously.21
Cortical lesions were manually segmented using median 7T T1w and T1 map MP2RAGE, T2*w GRE, and T2*w EPI images using Display (https://github.com/BIC-MNI/Display, Montreal Neurological Institute). Use of both T2*w GRE and EPI sequences allowed for assessment of cortical lesions even in cases in which not all T2*w GRE volumes were acquired or in which one of the sets of T2*w images was motion-degraded. Cortical lesions were segmented independently by two raters (ESB and JM), followed by consensus review. Lesions were categorized as leukocortical (type 1, involving cortex and white matter), intracortical (type 2, confined to the cortex and not touching the pial surface of the brain) and subpial (type 3, involving the cortex exclusively and touching the pial surface, and type 4, involving cortex and the white matter and touching the pial surface).2 Cortical lesions were hypointense on T1w MP2RAGE images and/or hyperintense on T2*w images and were seen on at least two consecutive axial slices. Cortical lesion volumes and median T1 within lesions, including the white matter portion of leukocortical lesions, were calculated.
Supplementary methods describe spinal cord, white matter, and chronic active white matter (phase rim, PRL) lesion segmentation, brain volume and spinal cord area measurements, and LME assessment.
Cortical segmentation
3T and 7T T1w images were linearly registered in MNI space (FLIRT), followed by white matter lesion filling. 7T whole-brain segmentation used spatially localized atlas network tiles (SLANT).22 Segmentation output was transformed to native 7T space to determine volume and median T1 of each of 100 cortical regions (50 per hemisphere) and volume of each cortical lesion within the cortical regions. Cortical regions were grouped by lobe and function (Supplementary Table 2).
Statistical analysis
Generalized linear models (GLM) determined the relationship of MRI measures and disease phenotype (relapsing-remitting versus progressive), adjusting for age and sex. For cortical and spinal cord lesion counts, a negative binomial distribution and log-link function were used. For cortical and white matter lesion volumes, volumes were log-transformed.
A mixed-effects model was used to determine the relationship between cortical lesions and regional cortical volume.
Pearson partial correlation coefficients evaluated the association between cortical lesion burden, PRL number, and spinal cord lesion number, with adjustment for age, sex, and white matter lesion volume following Box-Cox transformation of MRI variables.
Linear regression was used to determine the relationship between MRI variables (cortical lesion total and subtype volumes, white matter lesion volume, spinal cord lesion number, normalized brain volume) and disability measures, following Box-Cox transformation of MRI and disability variables. Two sets of covariates were used: (1) age; and (2) age, white matter lesion volume, and spinal cord lesion burden. Sex was not associated with MRI or disability variables so was not included as a covariate.
Statistical significance was set at p<0.05. P values for relationships between MRI and disability variables were adjusted for false discovery rate. See supplementary methods for additional details.
Results
Cortical lesions are more frequent in progressive MS
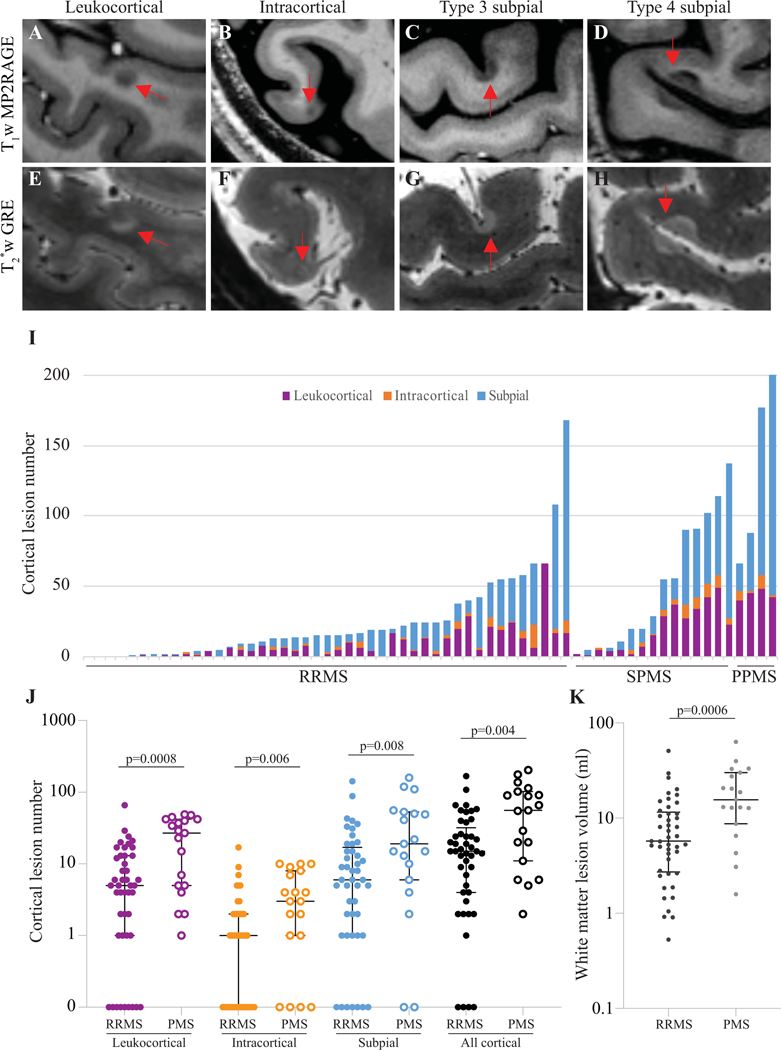
64 individuals with MS, including 45 with RRMS, 15 with SPMS, and 4 with PPMS, underwent clinical evaluation, 3T brain and spinal cord MRI, and 7T brain MRI (Table 1). Intraclass correlation coefficient between the two cortical lesion raters was 0.91 (95% confidence interval (CI) 0.85–0.94) for total cortical lesions, 0.95 (CI 0.91–0.97) for subpial lesions, and 0.93 (CI 0.88–0.95) for leukocortical lesions. 94% of individuals (60/64) had at least one cortical lesion, and 86% (55/64) had at least one subpial lesion (Fig. 1).
Table 1.
Demographics and disease characteristics
| RRMS, n=45 | PMS, n=19 | P value | |
|---|---|---|---|
| Female (%) | 28 (62) | 12 (63) | >0.99d |
| Age (years)a | 46 ± 10 (29–69) | 58 ± 10 (44–77) | <0.0001e |
| Disease duration (years)a | 10 ± 8 (0–32) | 25 ± 11 (7–42) | <0.0001e |
| Disease modifying therapy, n (%) | |||
| None | 10 (22) | 3 (16) | |
| Interferon | 6 (13) | 2 (11) | |
| Glatiramer acetate | 2 (4) | 1 (5) | |
| Dimethyl fumarate | 14 (31) | 4 (21) | |
| Fingolimod | 3 (7) | ||
| Teriflunomide | 1 (5) | ||
| Anti-CD20 | 2 (4) | 8 (42) | |
| Daclizumab | 4 (9) | ||
| Alemtuzumab | 1 (2) | ||
| Natalizumab | 3 (7) | ||
| Disability | |||
| EDSSb | 1.5, 1 (0–6.5) | 6, 2 (2–7.5) | <0.0001f |
| 25TW time (sec)c | 5.0 ± 2.8 | 13.7 ± 11.6 | <0.0001e |
| 9HPT time (sec)c | 19.6 ± 3.9 | 38.9 ± 17.8 | <0.0001e |
| SDMTc | 58 ± 11 | 34 ± 13 | <0.0001e |
| PASATc | 53 ± 7 | 42 ± 14 | <0.0001e |
Mean ± standard deviation (range).
Median, interquartile range (range).
Mean ± standard deviation.
Fisher’s exact test.
T-test.
Mann-Whitney test.
RRMS: relapsing remitting multiple sclerosis (MS). PMS: progressive MS. EDSS: expanded disability status scale. 25TW: 25-foot timed walk. 9HPT: 9-hole peg test. SDMT: symbol digit modality test. PASAT: paced auditory serial addition test. IQR: interquartile range.
Figure 1. Cortical lesions are present in most adults with MS and are more frequent in progressive MS.
Cortical lesions (arrows) were identified on T1 and T2*w images and categorized as leukocortical (A, E), intracortical (B, F), or subpial (C, D, G, H). (I) Total cortical lesion number and relative number of different cortical lesion subtypes (after grouping together both subpial lesion types) varied widely among participants. Leukocortical, intracortical, subpial, and total cortical lesion number (J), as well as white matter lesion volume (K), were higher in progressive versus relapsing MS.
T1w MP2RAGE: T1 weighted magnetization prepared 2 rapid gradient echoes. T2*w GRE: T2* weighted gradient-recalled echo. RRMS: relapsing remitting multiple sclerosis. SPMS: secondary progressive multiple sclerosis. PPMS: primary progressive multiple sclerosis. PMS: progressive multiple sclerosis.
For the purpose of future longitudinal studies assessing the dynamics of cortical lesions, the cohort was enriched for individuals with stable white matter lesions in the year prior to enrollment. There was no difference in age, disease duration, or disability measures between individuals with stable versus active relapsing MS (Supplementary Table 3). There were no differences in cortical lesion burden, white matter lesion volume, or brain volumes between these two groups. Thus, the stable versus active categorization was not considered in subsequent analyses presented here.
Cortical lesion number was higher in progressive (median 56, interquartile range (IQR) 91, range 2–203) than relapsing MS (median 15, IQR 28, range 0–168, p=0.004). Lesion number was higher for all cortical lesion subtypes in progressive versus relapsing MS (Table 2, Fig. 1I–K), as was white matter lesion volume. Spinal cord lesion number was also higher in progressive MS. After adjustment for age and sex, total cortical lesion number (p=0.0024), leukocortical lesion number (p=0.006) and volume (p=0.02), subpial lesion number (p=0.04), and spinal cord lesion number (p<0.0001) were higher in progressive MS; intracortical lesion number and white matter and cortical (except leukocortical) lesion volumes were not.
Table 2.
MRI measures in relapsing versus progressive multiple sclerosis cases.
| RRMS, n=45 | PMS, n=19 | P value | ||
|---|---|---|---|---|
| Adj for age and sexe | ||||
| Total cortical lesions a | ||||
| Lesion number | 15, 28 (0–168) | 56, 91 (2–203) | 0.004c | 0.0024 |
| Lesion volume (μL) | 384, 907 (0–9888) | 1268, 3298 (32–8918) | 0.021c | 0.14 |
| Leukocortical lesions a | ||||
| Lesion number | 5, 12 (0–66) | 27, 36 (1–49) | 0.0008c | 0.006 |
| Lesion volume (μL) | 102, 230 (0–2818) | 553, 948 (11–1698) | 0.003c | 0.020 |
| Intracortical lesions a | ||||
| Lesion number | 1, 2 (0–17) | 3, 7 (0–10) | 0.006c | 0.10 |
| Lesion volume (μL) | 4, 8 (0–65) | 9, 27 (0–71) | 0.035c | 0.18 |
| Subpial lesions a | ||||
| Lesion number | 6, 16 (0–142) | 19, 47 (0–159) | 0.008c | 0.043 |
| Lesion volume (μL) | 209, 719 (0–9660) | 760, 1900 (0–8015) | 0.033c | 0.50 |
| White matter lesion volume (ml) a | 5.7, 8.8 (0.5–50.8) | 15.6, 21.3 (1.6–63.1) | 0.0006c | 0.09 |
| Brain volume b | 1512 ± 98 | 1433 ± 69 | 0.003d | 0.17 |
| Gray matter volume b | 820 ± 103 | 759 ± 81 | 0.029d | 0.95 |
| Cortical volume b | 644 ± 82 | 594 ± 65 | 0.025d | 0.96 |
| White matter volume b | 692 ± 68 | 673 ± 57 | 0.31d | 0.05 |
| Spinal cord lesion number a | 4, 7 (0–16) | 11, 7 (1–19) | 0.0007c | <0.0001 |
Median, interquartile range (range).
Mean ± standard deviation.
Mann–Whitney test.
T-test.
Generalized linear model.
To determine whether individuals without high cortical lesion burden might have other explanations for a progressive MS disease course, we examined the 5/19 individuals with progressive MS (all SPMS) for whom cortical lesion number was below the median for the entire cohort. These individuals did not differ from the remaining progressive MS cohort in age, disease duration, white matter lesion volume, spinal cord lesion number, brain volume, spinal cord area, average cortical lesion T1, or any of the disability measures. However, spinal cord lesion number was in the top quartile for the cohort for 2/5 individuals, and white matter lesion volume was in the top quartile of the cohort for one additional individual.
Cortical lesions form in hotspots
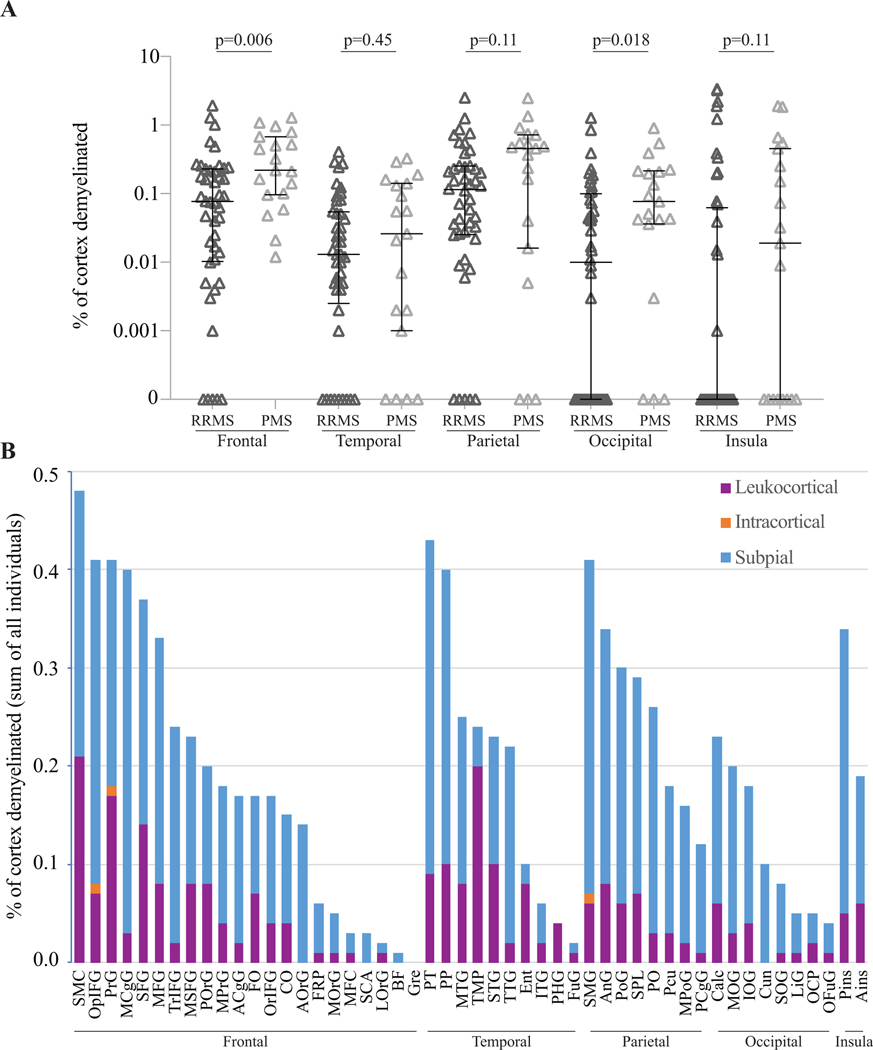
The average percentage of cortex occupied by cortical lesions was highest in the parietal lobe, followed by frontal, occipital, and temporal lobes, and insula. The trend was similar for subpial lesions. In the frontal and occipital lobes, percentage of cortex occupied by lesions was higher in individuals with progressive versus relapsing disease (Fig. 2A, Supplementary Table 4).
Figure 2. Cortical lesions are distributed unevenly across the brain but are more frequent in most anatomic regions in progressive MS.
(A) The average percentage of cortex occupied by cortical lesions was higher in progressive MS in the frontal and occipital lobes. (B) Cortical lesions were not distributed equally across 50 different regions. Subpial lesions were more frequent in some cortical regions, whereas leukocortical lesions predominately affected other cortical regions.
RRMS: relapsing remitting multiple sclerosis. PMS: progressive multiple sclerosis.
ACgG: anterior cingulate gyrus, Ains: anterior insula, AnG: angular gyrus, AOrG: anterior orbital gyrus, BF: basal forebrain, Calc: calcarine cortex, CO: central operculum, Cun: cuneus, Ent: entorhinal area, FO: frontal operculum, FRP: frontal pole, FuG: fusiform gyrus, Gre: gyrus rectus, IOG: inferior occipital gyrus, ITG: inferior temporal gyrus, LiG: lingual gyrus, LOrG: lateral orbital gyrus, MCgG: middle cingulate gyrus, MFC: medial frontal cortex, MFG: middle frontal gyrus, MOG: middle occipital gyrus, MOrG: medial orbital gyrus, MPoG: postcentral gyrus medial segment, MPrG: precentral gyrus medial segment, MSFG: superior frontal gyrus medial segment, MTG: middle temporal gyrus, OCP: occipital pole, OFuG: occipital fusiform gyrus, OpIFG: opercular part of the inferior frontal gyrus, OrIFG: orbital part of the inferior frontal gyrus, PCgG: posterior cingulate gyrus, PCu: precuneus, PHG: parahippocampal gyrus, Pins: posterior insula, PO: parietal operculum, PoG: postcentral gyrus, POrG: posterior orbital gyrus, PP: planum polare, PrG: precentral gyrus, PT: planum temporale, SCA: subcallosal area, SFG: superior frontal gyrus, SMC: supplementary motor cortex, SMG: supramarginal gyrus, SOG: superior occipital gyrus, SPL: superior parietal lobule, STG: superior temporal gyrus, TMP: temporal pole, TrIFG: triangular part of the inferior frontal gyrus, TTG: transverse temporal gyrus.
Since cortical lesions were sparsely distributed over the 50 cortical regions, we calculated the total cortical lesion volume in each region and divided it by the volume of the region summed across the entire cohort, giving the percentage of each region occupied by cortical lesions. Cortical lesions were not uniformly distributed (median 0.18%, IQR 0.21%, range 0–0.48%, Kolmogorov-Smirnov test for uniformity p=0.006) (Fig. 2B). The relative volumes of leukocortical, intracortical, and subpial lesions in each region were also variable (median percentage leukocortical 24%, IQR 20%, range 0–100%, p<0.0007). At the extremes, subpial lesions accounted for ≥90% of the cortical lesion volume in the cuneus, triangular part of the inferior frontal gyrus, middle cingulate gyrus, posterior cingulate gyrus, and transverse temporal gyrus, while the region with the highest percentage of leukocortical lesions by volume (84%) was the temporal pole. The percentage of individuals with lesions in each region was also highly variable (median 30%, IQR 30%, p=0.004), ranging from 75% of people with at least one cortical lesion in the superior frontal gyrus to 3% of people with at least one lesion in the subcallosal area (Supplementary Fig. 1).
We compared cortical lesion number in individuals with relapsing versus progressive disease in each cortical region in which at least 20 individuals had at least one lesion (22/50 regions). Lesion number was higher in progressive MS in the cuneus, middle occipital gyrus, and precentral gyrus (Supplementary Table 5).
Cortical lesion morphology and appearance are heterogeneous
Relative frequency of cortical lesion subtypes varied across the cohort (Fig. 1I). The percentage of cortical lesions that were subpial did not differ between individuals with relapsing versus progressive MS (mean 53%, standard deviation 29% versus 48 ± 24%, p=0.50), nor did it correlate with disability measures (EDSS, 25TW, 9HPT, PASAT, SDMT). We also observed differences in position of lesions with respect to the cortex-white matter junction. At the extremes, some individuals had predominantly juxtacortical lesions, with few other white matter or cortical lesions (Fig. 3A), whereas others had predominantly subpial lesions (Fig. 3B). Leukocortical lesions were also variable in appearance, with some being similarly hypointense on T1w images in both the cortical and white matter portions of the lesion (Fig. 3C), whereas others were more hypointense in the white matter portion of the lesion (Fig. 3D). We also observed lesions that appeared to primarily involve the juxtacortical U-fibers (Fig. 3E).
Figure 3. Cortical lesion morphology is heterogeneous.
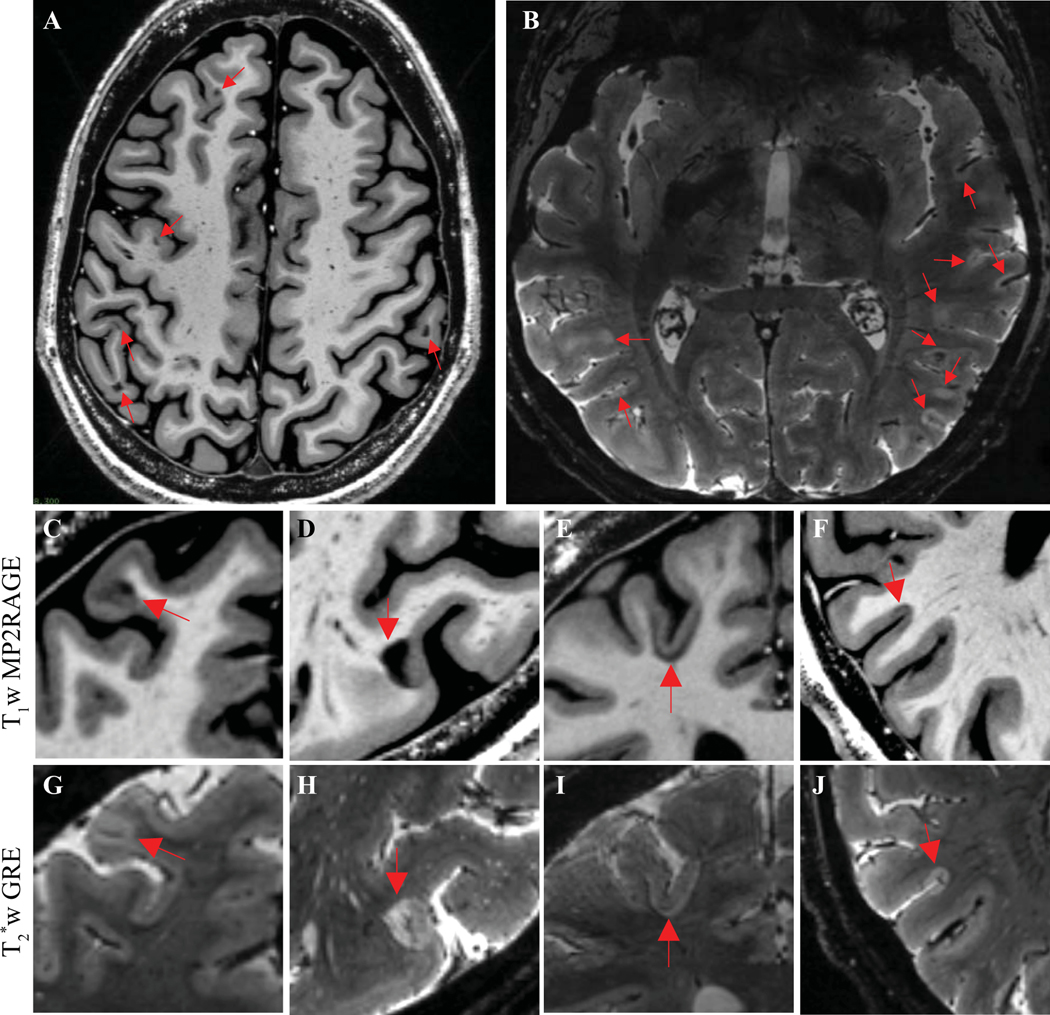
(A) Example of an individual with many juxtacortical lesions but few other white matter or cortical lesions, as seen on a representative T1w MP2RAGE image. (B) Example of an individual with many subpial lesions but few other cortical or white matter lesions, as seen on a representative T2*w image. (C, G) Example of a leukocortical lesion with similar signal change in the cortex and white matter portions of the lesion. (D, H) Example of a leukocortical lesion with marked signal change in the white matter portion of the lesion but more subtle signal change in the cortex, especially on T1w images. (E, I) Example of a lesion involving the juxtacortical U-fibers but relatively sparing the cortex. (F, J) Example of a subpial lesion that stops abruptly at the cortex-white matter border.
T1w MP2RAGE: T1 weighted magnetization prepared 2 rapid gradient echo. T2*w GRE: T2* weighted gradient-recalled echo.
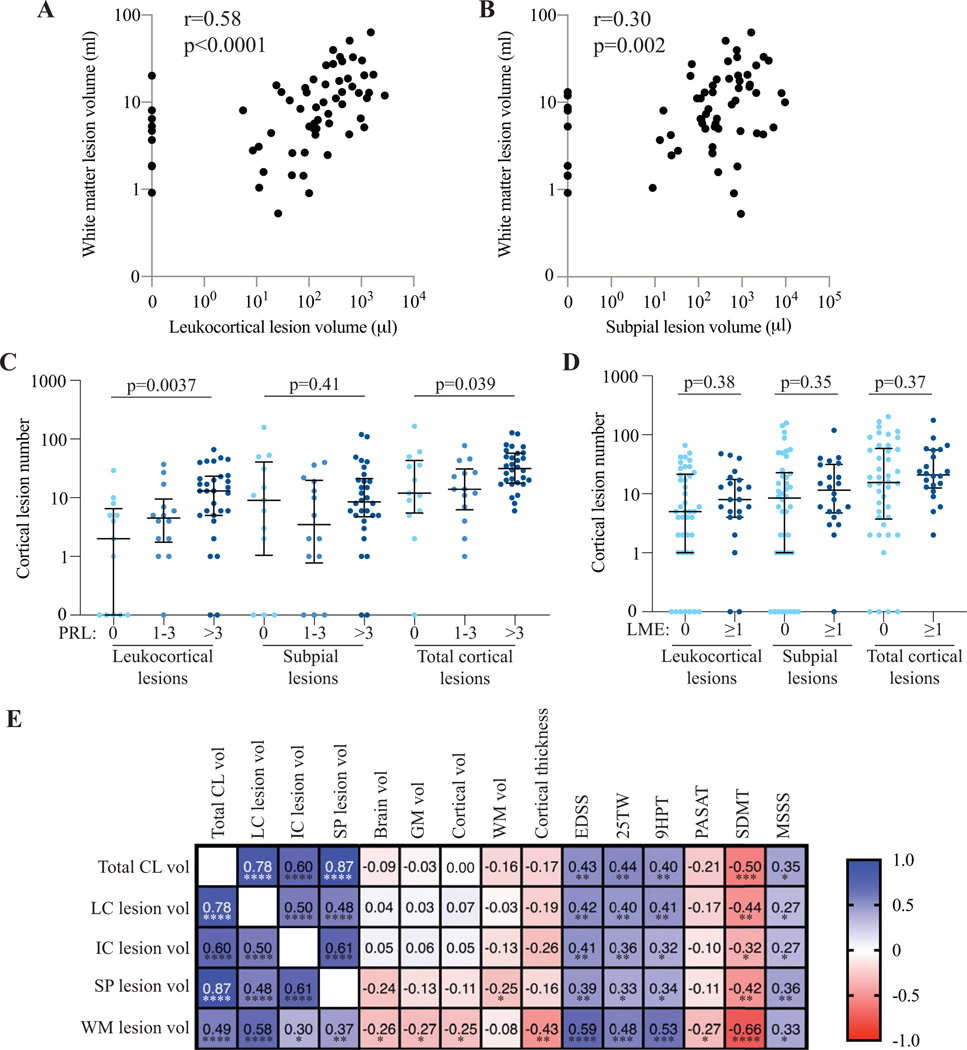
Leukocortical lesions are associated with total and chronic active white matter lesions
White matter lesion volume correlated strongly with leukocortical lesion volume, compared to moderate correlations with intracortical and subpial lesion volume (Fig. 4A, B, E). Cortical lesion number was higher in individuals with >3 white matter PRL versus those with 1–3 PRL versus those with no PRL (Fig. 4C, Supplementary Table 6). Results were similar for cortical lesion volume. Leukocortical and intracortical but not subpial lesion number were also higher in individuals with more PRL. After adjusting for age, sex, and white matter lesion volume, there was a significant association between PRL number and total cortical lesions and leukocortical lesions, but not with intracortical or subpial lesions.
Figure 4. Cortical lesion burden is associated with white matter lesion volume, white matter paramagnetic rim lesions, and disability measures, but not with leptomeningeal enhancement or brain volume.
White matter lesion volume is associated with leukocortical lesion volume (A) and subpial lesion volume (B). (C) Total cortical lesion and leukocortical lesion number, but not subpial lesion number, are higher in individuals with white matter PRL. (D) There is no difference in cortical lesion number in individuals with versus without LME. (E) Volume of total cortical lesions and cortical lesion subtypes are correlated with most measures of disability tested but not with brain volume, cortical volume, white matter volume, or cortical thickness. In general, the strongest associations are with SDMT.
PRL: paramagnetic rim lesion. LME: leptomeningeal enhancement. CL: cortical lesion. LC: leukocortical. IC: intracortical. SP: subpial. WM: white matter. GM: gray matter. EDSS: expanded disability status scale. 25TW: 25-foot timed walk. 9HPT: 9-hole peg test. PASAT: paced auditory symbol addition test. SDMT: symbol digit modality test. MSSS: multiple sclerosis severity scale. *: p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001. P values for correlations between MRI measures and disability measures are adjusted for false discovery rate.
Cortical lesion burden is not associated with leptomeningeal enhancement
16/58 individuals (27%) had at least one focus of LME. Cortical lesion number was not different in individuals with LME versus without (Fig. 4D, Supplementary Table 7), nor were there differences in cortical lesion volume, subtype number or volume, or white matter lesion volume. There was no correlation between LME number and cortical lesion number, cortical lesion volume, or white matter lesion volume. We examined the location of each of the 23 identified foci of LME. One focus of LME was adjacent to a subpial lesion. For an additional four foci, a cortical lesion (in two cases leukocortical and in two cases subpial) was located within the same sulcus as the LME. The remaining 18 foci of LME were not located near a cortical lesion.
Cortical lesion burden is not associated with brain volume
Except for a weak correlation between subpial lesion and white matter volume (r=−0.25, p=0.047), volume of total cortical lesions or cortical lesion subtypes did not correlate with total brain volume, total gray matter volume, cortical gray matter volume, white matter volume, or cortical thickness (Fig. 4E). There was also no relationship between cortical lesion volume in a given cortical region and the volume of that region (slope estimate 0.011, p=0.30).
Cortical lesion burden is associated with spinal cord lesion number but not area
Spinal cord lesion number was correlated with cortical lesion number and volume (Supplementary Table 8). A similar association was observed for each cortical lesion subtype. After adjusting for age, sex, and white matter lesion volume, the relationship between spinal cord and cortical lesions remained significant for total cortical lesions and for each cortical lesion subtype. There was no correlation between cortical lesion number or volume and spinal cord cross-sectional area.
Cortical lesion volume correlates with physical and cognitive disability
Total and subtype cortical lesion volumes correlated with EDSS, 25TW, 9HPT, and MSSS. There was no correlation between cortical lesion volume and PASAT, a cognitive test, but there was a correlation between cortical lesion volume and SDMT, which in the written form used here tests both cognitive and physical disability. White matter lesion volume was correlated with all tested disability measures (Fig. 4E). After adjusting for age, there was a significant relationship between cortical lesion volume and EDSS, 25TW, and SDMT (Supplementary Table 9). After adjusting for age, white matter lesion volume, and spinal cord lesion number, there were no significant associations between cortical lesion volume and disability measures.
We also assessed the association of cortical lesion volume in different functional areas with physical and cognitive disability (Supplementary Table 2). For cortical lesions and subtypes, associations with each disability measure were similar across functional areas (Supplementary Fig 2).
Cortical lesion T1 values
Median T1 was highest for leukocortical lesions, followed by type 4 subpial lesions, intracortical lesions, and type 3 subpial lesions (Supplementary Table 10). There was no difference in median cortical lesion T1 per individual between relapsing and progressive MS for any cortical lesion subtype. There was no association between median T1 and lesion number within cortical regions.
Discussion
We characterized cortical lesion frequency and location and found that some cortical regions are especially susceptible to cortical lesions. We also found that subpial lesions are not strongly correlated with total or chronic active white matter lesions but are associated with spinal cord lesions. Neither leukocortical nor subpial lesions are associated with LME. All subtypes of cortical lesions are more common in progressive MS, and cortical lesion volumes are correlated with measures of disability.
Relative to prior studies evaluating cortical lesions with 7T MRI, our cohort is almost twice as large,11, 12, 14 hopefully improving generalizability of our results. Previous 7T studies used either MPRAGE (with ≥0.7mm isotropic resolution) or T2*w images to evaluate cortical lesions, whereas we used a more sensitive T1w sequence (MP2RAGE), at higher resolution (0.5mm) and with combination of multiple acquisitions, together with T2*w images. Our interrater reliability was high compared to prior studies,11, 23 likely due to the improved visibility of cortical lesions on our images and the ability to verify subtle lesions on a second image type.
Our mapping of cortical lesions to cortical regions demonstrates nonuniform distribution of cortical lesions across the cortex, with some hotspots for cortical lesion formation. Our 7T scans often incompletely cover the lower temporal and occipital lobes, so our analysis likely underestimates the frequency of lesions in those regions. However, there are clear differences in lesion frequency even within well-imaged regions. Of the five cortical regions with the highest relative demyelination, two (supplementary motor cortex and precentral gyrus) are key for motor function. The other three (planum temporale, supramarginal gyrus, and opercular part of the inferior frontal gyrus) are all implicated in receptive language. While motor dysfunction is clearly important and prevalent in MS, some studies have also demonstrated language deficits.24, 25 Further investigation will be needed to understand if cortical lesions contribute to language dysfunction. In addition to the differences in total cortical lesion volume by cortical region, there are also differences in lesion subtype distribution. In some regions, most cortical lesions are subpial, whereas in other regions most cortical lesions are leukocortical. This may be due to differences in accessibility of inflammatory cells and mediators to those regions, or to intrinsic differences in the cortical tissues themselves, such as myelination patterns, glial phenotypes, and neuronal activity, which can regulate myelination in the developing cortex.26
Despite the relatively high sensitivity of our imaging methods, the percentage of cortex occupied by cortical lesions (median 0.16% in the frontal lobe) is very low compared to a previous systematic survey of 20 postmortem brains, in which a mean of 26.5% of the cortex was demyelinated.2 The relative contributions of MRI sensitivity versus differences in patient characteristics to this discrepancy are uncertain. Further MRI-pathology correlation studies will be needed to understand the true sensitivity of our methods and the extent of cortical demyelination in a contemporary MS population.
Given differences in myelination, inflammation, and repair between the cortex and other CNS tissue compartments,3, 26–28 as well as hypothesized differences in the source of inflammation in subpial lesions vs other types of MS lesions,4, 6, 29 we sought to understand the relationships between subpial lesions and white matter and spinal cord lesions. We find that subpial lesion volume correlates only moderately with white matter lesion volume and that subpial lesions, unlike leukocortical lesions, are not associated with chronic active white matter lesions. These findings, in combination with our observations that there are often stark differences in lesion intensity in the cortical versus white matter portions of leukocortical lesions as well as lesions that stop abruptly at the cortex-white matter junction (on either side), suggest differences in inflammatory or repair mechanisms, or in susceptibility to damage, in cortex and white matter. This is consistent with postmortem pathology studies demonstrating few inflammatory cells in chronic cortical lesions, even in leukocortical lesions in which there are macrophages/microglia in the white matter portion of the lesion.27, 30, 31 Further studies will be required to better understand the relationship between chronic meningeal inflammation overlying subpial lesions4, 6 and chronic white matter inflammation.
We observe a significant association between spinal cord lesions and both leukocortical and subpial lesions, even after adjusting for white matter lesions, consistent with observations of cases in which there is high cortical and spinal cord lesion burden but low white matter lesion burden,32 and suggesting a potential shared mechanism of lesion formation, perhaps related to proximity to the meninges. Longitudinal and mechanistic studies will be important for understanding how lesion repair and growth differ in cortex versus white matter and spinal cord.
Despite the evidence that meningeal inflammation leads to cortical demyelination, we find a lack of association between cortical lesions and LME. Although the frequency of LME, assessed using 3T images, is lower than in recent 7T studies,33, 34 we and others have observed that cortical lesions are much more frequent than LME, and LME is rarely found in close proximity to cortical lesions. Prior radiological-pathological correlation showed evidence of meningeal inflammation in areas of LME,35 however our findings suggest a non-straightforward relationship between LME and the meningeal inflammatory aggregates often observed overlying subpial lesions in pathology studies.
We did not find an association between cortical lesion volume and cortical volume or thickness, either globally or within individual cortical regions. While studies at 3T have demonstrated an association between juxtacortical/cortical lesions and cortical thinning, a recent 7T study found no difference in cortical thickness between people with high versus low cortical lesion burden.36–38 Given the variability in cortical volume and thickness across individuals, longitudinal studies examining changes in cortical volume over time will be necessary to better understand how cortical lesions contribute to cortical atrophy.
On average, people with progressive MS had more cortical lesions, but several individuals in our cohort with SPMS had few cortical lesions, not all of whom had high white matter or spinal cord lesion burden. Thus, it may be that either severe lesion burden in any one of the CNS tissue types or the sum of damage to the CNS is important in determining progression. Alternatively, one or a few lesions in a particularly eloquent region may trigger disability or progressive symptoms. Longitudinal studies will be necessary to understand when cortical lesions form and whether their formation early in disease leads to disability progression later on or whether, as several studies have suggested, cortical lesion formation continues during progressive phases of the disease.12, 39
While each cortical lesion subtype was associated with multiple measures of disability, there were no significant associations between cortical lesion volume and disability measures after adjusting for age, white matter lesions, and spinal cord lesions. This finding highlights that, despite potential tissue-specific mechanisms of lesion formation and repair, it is difficult to tease out whether and how different lesion types contribute independently to disability. Larger, longitudinal studies will also be needed to fully understand these relationships, which may in turn help determine which aspects of MS pathology should be targeted for treatment in which patients.
Supplementary Material
Acknowledgments
We acknowledge the staff of the National Institute of Neurological Disorders and Stroke Neuroimmunology Clinic for care of and collection of clinical data from the study participants and the staff of the National Institute of Mental Health Functional MRI Facility, Matthew Schindler, and Martina Absinta for help with MRI acquisitions.
Funding
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health. Erin Beck was supported by a Clinician Scientist Development Award and a Career Transition Fellowship Award from the National Multiple Sclerosis Society.
Footnotes
Conflicting interests
The authors declare that there is no conflict of interest.
References
- 1.Brownell B and Hughes JT. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry 1962; 25: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bo L, Vedeler CA, Nyland HI, et al. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol 2003; 62: 723–732. [DOI] [PubMed] [Google Scholar]
- 3.Lucchinetti CF, Popescu BF, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 2011; 365: 2188–2197. DOI: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011; 134: 2755–2771. DOI: 10.1093/brain/awr182. [DOI] [PubMed] [Google Scholar]
- 5.Magliozzi R, Howell OW, Reeves C, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 2010; 68: 477–493. DOI: 10.1002/ana.22230. [DOI] [PubMed] [Google Scholar]
- 6.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007; 130: 1089–1104. DOI: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 7.Seewann A, Kooi EJ, Roosendaal SD, et al. Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology 2012; 78: 302–308. DOI: 10.1212/WNL.0b013e31824528a0. [DOI] [PubMed] [Google Scholar]
- 8.Kilsdonk ID, Jonkman LE, Klaver R, et al. Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post-mortem verification study. Brain 2016; 139: 1472–1481. DOI: 10.1093/brain/aww037. [DOI] [PubMed] [Google Scholar]
- 9.Sethi V, Yousry TA, Muhlert N, et al. Improved detection of cortical MS lesions with phase-sensitive inversion recovery MRI. J Neurol Neurosurg Psychiatry 2012; 83: 877–882. 2012/07/19. DOI: 10.1136/jnnp-2012-303023. [DOI] [PubMed] [Google Scholar]
- 10.Mainero C, Benner T, Radding A, et al. In vivo imaging of cortical pathology in multiple sclerosis using ultra-high field MRI. Neurology 2009; 73: 941–948. DOI: 10.1212/WNL.0b013e3181b64bf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison DM, Roy S, Oh J, et al. Association of Cortical Lesion Burden on 7-T Magnetic Resonance Imaging With Cognition and Disability in Multiple Sclerosis. JAMA Neurol 2015; 72: 1004–1012. DOI: 10.1001/jamaneurol.2015.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treaba CA, Granberg TE, Sormani MP, et al. Longitudinal Characterization of Cortical Lesion Development and Evolution in Multiple Sclerosis with 7.0-T MRI. Radiology 2019; 291: 740–749. 2019/04/09. DOI: 10.1148/radiol.2019181719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louapre C, Govindarajan ST, Giannì C, et al. The association between intra- and juxta-cortical pathology and cognitive impairment in multiple sclerosis by quantitative T. Neuroimage Clin 2016; 12: 879–886. 2016/11/03. DOI: 10.1016/j.nicl.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen AS, Kinkel RP, Madigan N, et al. Contribution of cortical lesion subtypes at 7T MRI to physical and cognitive performance in MS. Neurology 2013; 81: 641–649. 2013/07/17. DOI: 10.1212/WNL.0b013e3182a08ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mistry N, Abdel-Fahim R, Mougin O, et al. Cortical lesion load correlates with diffuse injury of multiple sclerosis normal appearing white matter. Mult Scler 2014; 20: 227–233. 2013/07/17. DOI: 10.1177/1352458513496344. [DOI] [PubMed] [Google Scholar]
- 16.Beck ES, Sati P, Sethi V, et al. Improved Visualization of Cortical Lesions in Multiple Sclerosis Using 7T MP2RAGE. AJNR Am J Neuroradiol 2018. 2018/02/14. DOI: 10.3174/ajnr.A5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. 2011/03/10. DOI: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roxburgh RH, Seaman SR, Masterman T, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology 2005; 64: 1144–1151. 2005/04/13. DOI: 10.1212/01.WNL.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson M and Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001; 5: 143–156. 2001/08/23. DOI: 10.1016/s1361-8415(01)00036–6. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002; 17: 825–841. 2002/10/16. DOI: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 21.Maranzano J, Dadar M, Rudko DA, et al. Comparison of Multiple Sclerosis Cortical Lesion Types Detected by Multicontrast 3T and 7T MRI. AJNR Am J Neuroradiol 2019; 40: 1162–1169. 2019/06/22. DOI: 10.3174/ajnr.A6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huo Y, Xu Z, Xiong Y, et al. 3D whole brain segmentation using spatially localized atlas network tiles. Neuroimage 2019; 194: 105–119. 2019/03/27. DOI: 10.1016/j.neuroimage.2019.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen AS, Kinkel RP, Tinelli E, et al. Focal cortical lesion detection in multiple sclerosis: 3 Tesla DIR versus 7 Tesla FLASH-T2. J Magn Reson Imaging 2012; 35: 537–542. DOI: 10.1002/jmri.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandstadter R, Fabian M, Leavitt VM, et al. Word-finding difficulty is a prevalent disease-related deficit in early multiple sclerosis. Mult Scler 2020; 26: 1752–1764. 2019/11/20. DOI: 10.1177/1352458519881760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renauld S, Mohamed-Said L and Macoir J. Language disorders in multiple sclerosis: A systematic review. Mult Scler Relat Disord 2016; 10: 103–111. 2016/12/07. DOI: 10.1016/j.msard.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Timmler S and Simons M. Grey matter myelination. Glia 2019; 67: 2063–2070. 2019/03/13. DOI: 10.1002/glia.23614. [DOI] [PubMed] [Google Scholar]
- 27.Bo L, Vedeler CA, Nyland H, et al. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler 2003; 9: 323–331. 2003/08/21. DOI: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- 28.Strijbis EMM, Kooi EJ, van der Valk P, et al. Cortical Remyelination Is Heterogeneous in Multiple Sclerosis. J Neuropathol Exp Neurol 2017; 76: 390–401. 2017/05/19. DOI: 10.1093/jnen/nlx023. [DOI] [PubMed] [Google Scholar]
- 29.Magliozzi R, Howell OW, Nicholas R, et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol 2018; 83: 739–755. 2018/03/09. DOI: 10.1002/ana.25197. [DOI] [PubMed] [Google Scholar]
- 30.Peterson JW, Bo L, Mork S, et al. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 2001; 50: 389–400. [DOI] [PubMed] [Google Scholar]
- 31.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016; 126: 2597–2609. 2016/06/09. DOI: 10.1172/JCI86198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trapp BD, Vignos M, Dudman J, et al. Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: a retrospective study. Lancet Neurol 2018; 17: 870–884. 2018/08/26. DOI: 10.1016/S1474-4422(18)30245-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zurawski J, Tauhid S, Chu R, et al. 7T MRI cerebral leptomeningeal enhancement is common in relapsing-remitting multiple sclerosis and is associated with cortical and thalamic lesions. Mult Scler 2020; 26: 177–187. 2019/11/13. DOI: 10.1177/1352458519885106. [DOI] [PubMed] [Google Scholar]
- 34.Ighani M, Jonas S, Izbudak I, et al. No association between cortical lesions and leptomeningeal enhancement on 7-Tesla MRI in multiple sclerosis. Mult Scler 2020; 26: 165–176. 2019/10/02. DOI: 10.1177/1352458519876037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Absinta M, Vuolo L, Rao A, et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology 2015; 85: 18–28. DOI: 10.1212/WNL.0000000000001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treaba CA, Herranz E, Barletta VT, et al. The relevance of multiple sclerosis cortical lesions on cortical thinning and their clinical impact as assessed by 7.0-T MRI. J Neurol 2021; 268: 2473–2481. 2021/02/02. DOI: 10.1007/s00415-021-10400-4. [DOI] [PubMed] [Google Scholar]
- 37.Pareto D, Sastre-Garriga J, Auger C, et al. Juxtacortical Lesions and Cortical Thinning in Multiple Sclerosis. AJNR Am J Neuroradiol 2015; 36: 2270–2276. 2015/10/10. DOI: 10.3174/ajnr.A4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geisseler O, Pflugshaupt T, Bezzola L, et al. The relevance of cortical lesions in patients with multiple sclerosis. BMC Neurol 2016; 16: 204. 2016/10/23. DOI: 10.1186/s12883-016-0718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sethi V, Yousry T, Muhlert N, et al. A longitudinal study of cortical grey matter lesion subtypes in relapse-onset multiple sclerosis. J Neurol Neurosurg Psychiatry 2016; 87: 750–753. DOI: 10.1136/jnnp-2015-311102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.