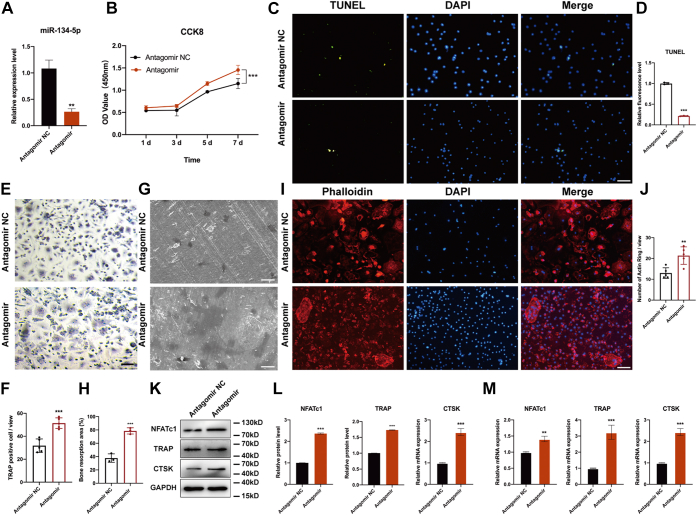
Figure 4.
miR-134-5p knockdown facilitates the osteoclast differentiation of BMMs.A, miR-134-5p antagomir or its negative control antagomir NC was transfected into BMMs, and the BMMs were then cultured with 30 ng/ml M-CSF and 50 mg/ml RANKL for 7 days to induce their differentiation into osteoclasts. The transfection efficiency was analyzed by qRT-PCR. B, the cell viability of BMMs transfected with antagomir or its control was determined by CCK-8 assay and measured under a microplate reader. C and D, cell apoptosis was determined with a TUNEL kit, and images were observed under a fluorescence microscope. The scale bars represent 100 μm. E and F, TRAP staining was performed for the analysis of osteoclast formation activity. The number of TRAP+ multinuclear osteoclasts was counted. The scale bars represent 100 μm. G and H, bone resorption ability was screened on bone slices through scanning electron microscope and bone resorption area was quantified with ImageJ software. The scale bars represent 50 μm. I and J, BMMs were induced to differentiate into osteoclasts and stained with phalloidin for the evaluation of actin ring formation. The number of F-actin rings per field was counted. The scale bars represent 100 μm. K and L, the protein expression of TRAP, CTSK, and NFATc1 was analyzed by Western blot. Semiquantitative analysis was conducted using ImageJ software. GAPDH was used as an internal reference control. M, quantitative RT-PCR was performed to measure the mRNA expression levels of TRAP, CTSK, and NFATc1. β-Actin was used as an internal reference gene. All data were expressed as means ± SD. ∗∗p < 0.01, ∗∗∗p < 0.001. BMMs, bone marrow macrophages; CCK-8, cell counting kit-8.