Fig 1. Multivalent minibinders exhibit very slow dissociation rates upon binding to the prefusion SARS-CoV-2-S glycoprotein trimer.
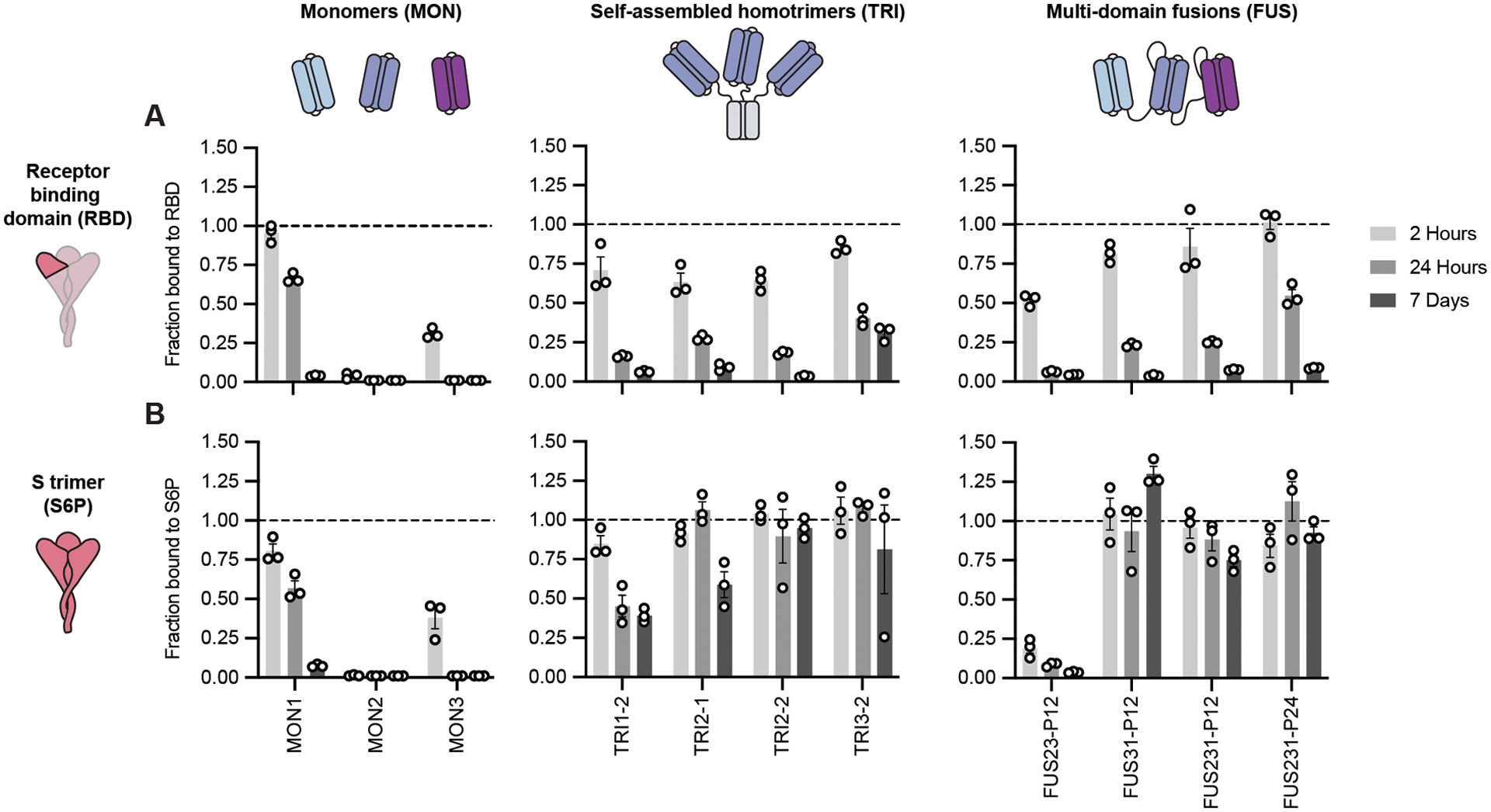
Dissociation of the minibinder construct was monitored by competition with 100-fold molar excess of untagged MON1 using AlphaLISA (Mean ± SEM, n = 3 technical replicates from a single experiment). (A) Dissociation was measured for indicated minibinder constructs complexed with the receptor-binding domain of SARS-CoV-2 (RBD). (B) Dissociation was measured for the indicated minibinder constructs complexed with the S trimer (S6P).
